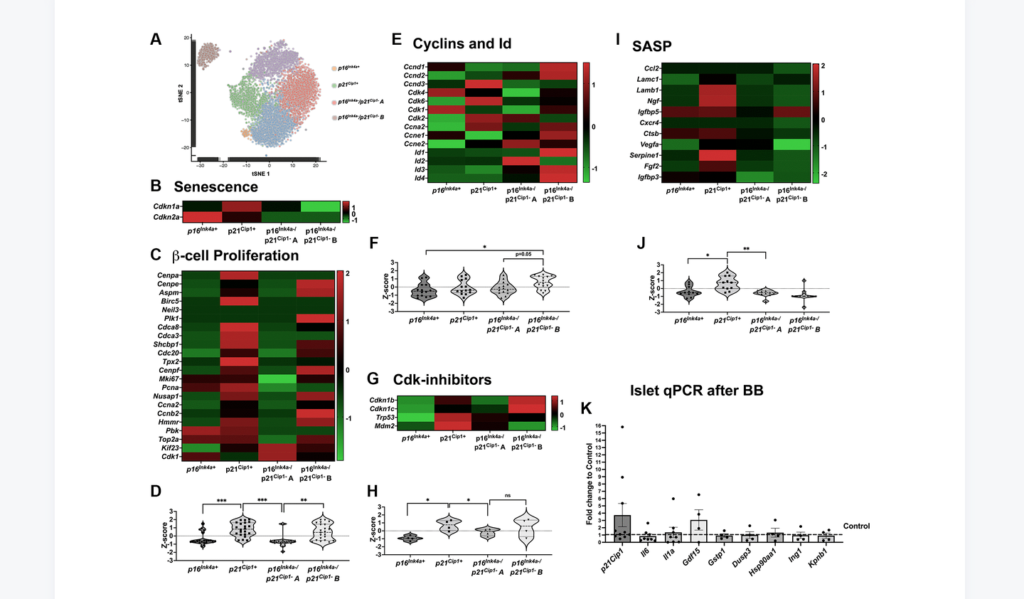
“[…] we set out to explore the effects of removing p16Ink4a+ senescent cells on the proliferative capacity and mass of β-cells […].”
BUFFALO, NY- January 31, 2023 – A new research paper was published on the cover of Aging (listed as “Aging (Albany NY)” by Medline/PubMed and “Aging-US” by Web of Science) Volume 15, Issue 2, entitled, “Clearance of p16Ink4a-positive cells in a mouse transgenic model does not change β-cell mass and has limited effects on their proliferative capacity.”
Type 2 diabetes is partly characterized by decreased β-cell mass and function which have been linked to cellular senescence. Despite a low basal proliferative rate of adult β-cells, they can respond to growth stimuli, but this proliferative capacity decreases with age and correlates with increased expression of senescence effector, p16Ink4a.
In a new study, researchers Nadine Bahour, Lucia Bleichmar, Cristian Abarca, Emeline Wilmann, Stephanie Sanjines, and Cristina Aguayo-Mazzucato from the Joslin Diabetes Center at Harvard Medical School hypothesized that selective deletion of p16Ink4a-positive cells would enhance the proliferative capacity of the remaining β-cells due to the elimination of the local senescence-associated secretory phenotype (SASP).
“We aimed to investigate the effects of p16Ink4a-positive cell removal on the mass and proliferative capacity of remaining β-cells using INK-ATTAC mice as a transgenic model of senolysis.”
Clearance of p16Ink4a-positive subpopulation was tested in mice of different ages, males and females, and with two different insulin resistance models: high-fat diet (HFD) and insulin receptor antagonist (S961). Clearance of p16Ink4a-positive cells did not affect the overall β-cell mass. β-cell proliferative capacity negatively correlated with cellular senescence load and clearance of p16Ink4a positive cells in 1-year-old HFD mice improved β-cell function and increased proliferative capacity in a subset of animals. Single-cell sequencing revealed that the targeted p16Ink4a subpopulation of β-cells is non-proliferative and non-SASP producing whereas additional senescent subpopulations remained contributing to continued local SASP secretion.
“In conclusion, deletion of p16Ink4a cells did not negatively impact beta-cell mass and blood glucose under basal and HFD conditions and proliferation was restored in a subset of HFD mice opening further therapeutic targets in the treatment of diabetes.”
DOI: https://doi.org/10.18632/aging.204483
Corresponding Author: Cristina Aguayo-Mazzucato – [email protected]
Keywords: beta cells, mass, proliferation, senolysis, senescence
Sign up for free Altmetric alerts about this article: https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.204483
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
About Aging-US:
Launched in 2009, Aging (Aging-US) publishes papers of general interest and biological significance in all fields of aging research and age-related diseases, including cancer—and now, with a special focus on COVID-19 vulnerability as an age-dependent syndrome. Topics in Aging go beyond traditional gerontology, including, but not limited to, cellular and molecular biology, human age-related diseases, pathology in model organisms, signal transduction pathways (e.g., p53, sirtuins, and PI-3K/AKT/mTOR, among others), and approaches to modulating these signaling pathways.
Please visit our website at www.Aging-US.com and connect with us:
For media inquiries, please contact [email protected].