“[…] [voluntary wheel running] differentially alters bone parameters depending on body weight […]”
BUFFALO, NY- February 8, 2023 – A new research paper was published in Aging (listed as “Aging (Albany NY)” by MEDLINE/PubMed and “Aging-US” by Web of Science) Volume 15, Issue 2, entitled, “Body weight influences musculoskeletal adaptation to long-term voluntary wheel running during aging in female mice.”
Aging increases the prevalence of sarcopenia and osteoporosis that are often both components of a musculoskeletal syndrome, osteosarcopenia. Osteosarcopenia is highly associated with frailty, falls, fractures, and disability, leading to decreased quality of life and increased morbidity and mortality. Frailty is the hallmark of aging that can be delayed with exercise.
In this new research paper, researchers Yukiko Kitase, Julian A. Vallejo, Sarah L. Dallas, Yixia Xie, Mark Dallas, LeAnn Tiede-Lewis, David Moore, Anthony Meljanac, Corrine Kumar, Carrie Zhao, Jennifer Rosser, Marco Brotto, Mark L. Johnson, Ziyue Liu, Michael J. Wacker, and Lynda Bonewald from Indiana University, University of Missouri and University of Texas wrote that their recent studies were initiated based on the hypothesis that long-term voluntary wheel running (VWR) in female mice from 12 to 18 or 22 months of age would have beneficial effects on the musculoskeletal system.
“Frequently osteoporosis and sarcopenia occur concurrently. It is not known if one precedes the other or if one condition influences disease progression of the other condition [26, 27]. We hypothesized that long-term voluntary exercise started later in life (12 months of age) would improve both skeletal muscle and bone parameters in aging female mice up to 22 months.”
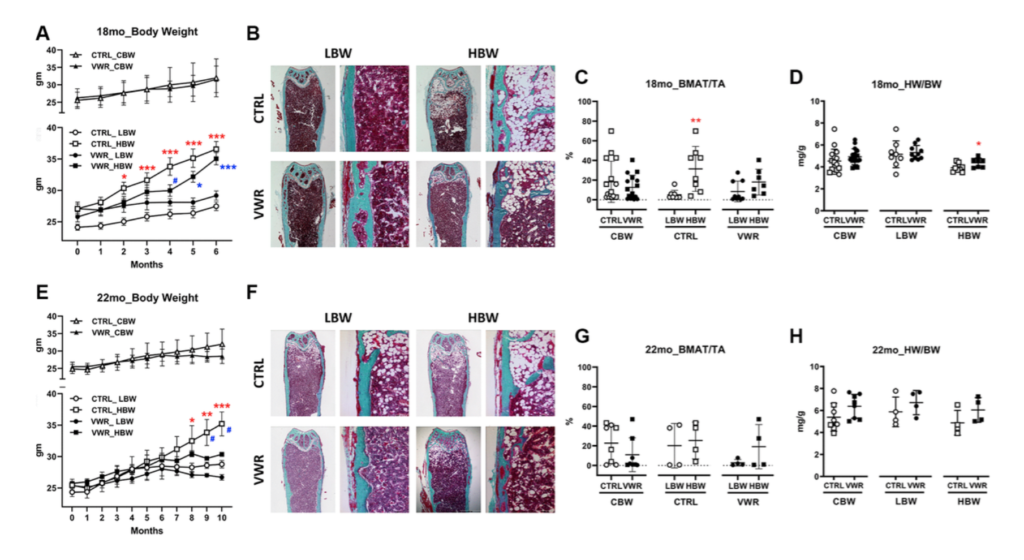
Mice were separated into high (HBW) and low (LBW) body weight based on final body weights upon termination of experiments. Bone marrow fat was significantly higher in HBW than LBW under sedentary conditions, but not with VWR. HBW was more protective for soleus size and function than LBW under sedentary conditions, however VWR increased soleus size and function regardless of body weight.
VWR plus HBW was more protective against muscle loss with aging. Similar effects of VWR plus HBW were observed with the extensor digitorum longus, EDL, however, LBW with VWR was beneficial in improving EDL fatigue resistance in 18 mo mice and was more beneficial with regards to muscle production of bone protective factors. VWR plus HBW maintained bone in aged animals.
In summary, HBW had a more beneficial effect on muscle and bone with aging especially in combination with exercise. These effects were independent of bone marrow fat, suggesting that intrinsic musculoskeletal adaptions were responsible for these beneficial effects.”
“Collectively, VWR has beneficial effects on bone health during advanced aging regardless of body weight, but VWR differentially alters bone parameters depending on body weight, with modifications in mechanical properties in LBW but structural modifications in HBW contributing to the prevention of osteopenia.”
Read the Full Paper: DOI: https://doi.org/10.18632/aging.204390
Corresponding Authors: Lynda Bonewald, Michael J. Wacker
Corresponding Emails: [email protected], [email protected]
Keywords: body weight, musculoskeletal adaptation, long-term, voluntary wheel running, aging
Sign up for free Altmetric alerts about this article: https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.204390
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
About Aging-US:
Launched in 2009, Aging (Aging-US) publishes papers of general interest and biological significance in all fields of aging research and age-related diseases, including cancer—and now, with a special focus on COVID-19 vulnerability as an age-dependent syndrome. Topics in Aging go beyond traditional gerontology, including, but not limited to, cellular and molecular biology, human age-related diseases, pathology in model organisms, signal transduction pathways (e.g., p53, sirtuins, and PI-3K/AKT/mTOR, among others), and approaches to modulating these signaling pathways.
Please visit our website at www.Aging-US.com and connect with us:
For media inquiries, please contact [email protected].