Dr. Wenbo Yu, Stanley from the Centre for Cancer Biology, SA Pathology and University of South Australia details a research perspective he co-authored that was published by Aging (Aging-US) in Volume 15, Issue 17, entitled, “A Poisson distribution-based general model of cancer rates and a cancer risk-dependent theory of aging.”
Behind the Study is a series of transcribed videos from researchers elaborating on their recent studies published by Aging (Aging-US). Visit the Aging (Aging-US) YouTube channel for more insights from outstanding authors.
—
Hello, I’m Wenbo Yu, Stanley, a scientist from Centre for Cancer Biology, Adelaide, Australia. I’m also affiliated with University of Adelaide and the University of South Australia. I’m interested in both translational and theoretical aspects of oncology. I’m glad to introduce my recent theoretical paper, “A Poisson distribution-based general model of cancer rates and a cancer risk-dependent theory of aging.”
I’m always puzzled by the complicated pathway of oncogenesis. But if we take a broad perspective that consider all cancers statistically and regressively, it allows me to set up a super simple model to correlate cancer incidents with the random mutations accumulated in cellular genome by using classic Poisson distribution.
It began in July 2021, during the COVID lockdown in Adelaide. When I had nothing to do at home, I started some calculation in my bedroom and then developed it over time. The results have led me to establish a connection between cancer incidence and aging, and finally, I developed a hypothesis of aging based on it.
We know that cancer is a disease of DNA. We also know that older age is the main risk factor for cancer. This largely reflects DNA damage within cells over time. However, for the increased cancer incidence with aging, there are alternative hypothesis. One is the disposable soma theory. This theory suggests that the wear and tear on the body is effectively maintained until after the reproduction period, because the selective forces decline after that period.
When applying this theory to cancer, it is believed that cancer stem cells were once suppressed, but later emerged, because our body are no longer effectively protected after the end of the reproduction period. However, if I assume that cancer incidence is only proportionally related to genome mutations, which accumulates randomly and constantly over time, I can model the incidence of cancer with a Poisson distribution. This results in S curve, which is so much higher than the real observed cancer rates. This is the model that only considered accumulated mutations without considering relaxed body protection. This calculation allows me to make the assumption that our bodies are not abandoned, but protected during the aging process.
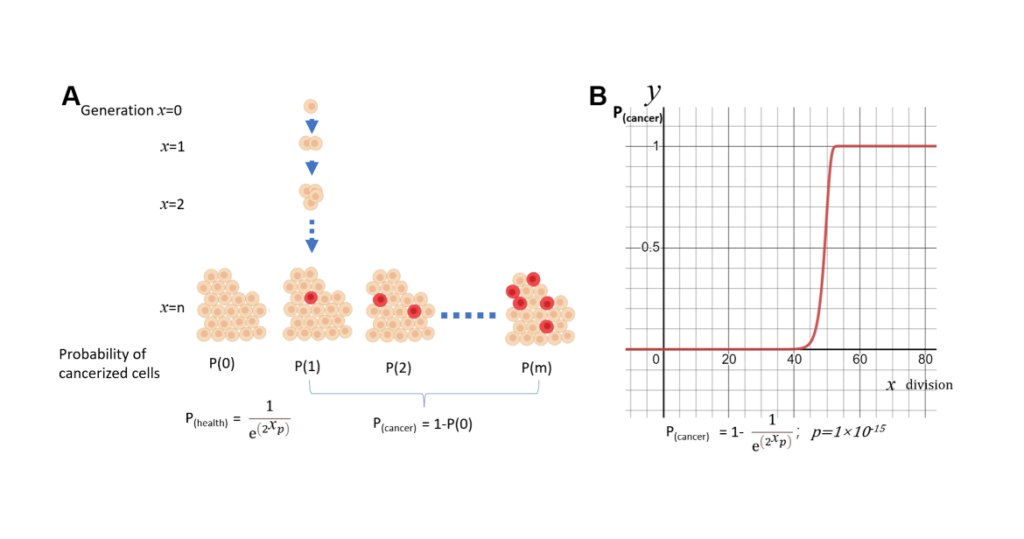
As we know, the probability from Poisson distribution is determined by N and P, with N representing the effective number of cell turnover, and the P representing the probability of single-cell transformation. Many studies found that as people age, cell division and turnover rates decrease. This gave us the eureka that if we consider reduction in cell turnover due to aging, we can make the model accurately predict the observed incidence of cancer in humans. So I call this model Not Poisson model, or NP model. Not derived from N, the turnover cell number. And Poisson derived from P, the probability of cancer within individual cell.
OK, now what’s the information we can get from this model? First, cancer development is ultimately inevitable. Second, aging reduces that cell turnover, which elongates cancer-free lifespan. The inspiration from this information lead me to the fundamental question of why we are mortal. As no theory has yet provided a satisfactory explanation for the phenomenon of aging, there is an ongoing debate as to whether aging is caused by imperfect evolution, or programmed survival strategies, or simply a result of the inevitable law of nature.
In this theory of aging, cancer is described as the inevitable increase of information entropy in cells. The key points of the theory are here.
First, aging is a phenomenon of systematical accumulation of positive entropy, which is determined by the second law of thermodynamics.
Second, the positive entropy can be resolved by cell division at a cellular level.
Third, however, the positive entropy cannot be completely resolved at the DNA level, which can be measured as information entropy. The increased information entropy ultimately leads to cancer.
Fourth, as a result, the inevitability of cancer limits the total number of cells that an individual organism can use in their lifespan. This limitation is the fundamental reason why we are mortal.
Aging is the result of insufficient cellular proliferation, which leads to the gradual inability to reconcile the positive entropy in our body. Human’s elongated aging is a wise evolutionary strategy to use our limited cell resources for longevity.
Many scientists believe that biological systems have the inherent ability to repair damage and replace defective cells, which suggests that they are not necessarily destined to die. Our very existence from the first cell on earth demonstrates that cells can renew themselves indefinitely. So the eternity of cells is not a problem. The problem is the eternity of the body.
This theory of information entropy predicts that any multicellular system will eventually develop cancer. The only way to overcome this limitation is through single colony selection, and the process of reproduction is just such a form of single colony selection. Natural elimination of imperfect seeds maintains the stability of information entropy from generation to generation.
We have further advanced our theory by introducing the concept of the impossible trilemma, which states that it is impossible to have all three of the following system components constant at the same time. First, structure. Second, information. And third, metabolism. These three phenomenon support each other. However, compromising at least one of these aspects becomes inevitable when the other two needs to be sustained.
From a translational view of this theory, one concept I’m quite interested now is the molecular clock. To prevent specific cancers, one hypothesis approach could be to manipulate the molecular clock in the tissue. If we can identify the molecular clock that regulates this particular tissue, we could slow down the turnover of stem cells in that tissue. For example, if we slow down the stem cell turnover in breast tissue, based on the theory, we could expect the delay of the onset of breast cancer. If there is any funding opportunity in future, I’d like to do some work as proof of concept.
Thanks to the co-author, Tessa Gargett, from Centre for Cancer Biology, and Zhenglong Du, from University of Adelaide. They actively participated in the discussion of this manuscript. And thanks to the audience.
Click here to read the full study published by Aging (Aging-US).
—
Aging (Aging-US) is an open-access journal that publishes research papers bi-monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].