Dr. Chang-Yi Cui and Alexandra G. Zonnefeld from the Laboratory of Genetics and Genomics, National Institute on Aging in Baltimore, MD, discuss a research paper they co-authored that was published by Aging (Aging-US) in Volume 16, Issue 8, entitled, “Characterization of age-associated gene expression changes in mouse sweat glands.”
Behind the Study is a series of transcribed videos from researchers elaborating on their recent studies published by Aging (Aging-US). Visit the Aging (Aging-US) YouTube channel for more insights from outstanding authors.
—
Hello, my name is Alexandra Zonnefeld, and I’m a postbaccalaureate fellow in the Laboratory of Genetics and Genomics in the National Institute on Aging. I’m a co-first author of our paper entitled, “Characterization of age-associated gene expression changes in mouse sweat glands“, which was recently published in the journal Aging.
Hello, I am Chang-Yi Cui, a Senior Associate Scientist from the same lab, and I’m a co-corresponding author of this paper.
Thermoregulation, the process of maintaining body temperature within a narrow range, is crucial for homeostasis. However, thermoregulation tends to decline with age leading to increased vulnerability to heat-related illness. In the United States alone, over 1,000 people die every summer from heatstroke with the majority being older individuals. Sweat glands play a major role in human thermoregulation as sweat evaporates from the skin’s surface effectively dissipating body heat.
Sweating capacity is known to decline with age. Our study confirms this, finding a significant reduction in active sweat gland number in older mice. However, the molecular mechanisms of this decline remain poorly characterized. To address this gap, we examined the gene expression programs in sweat glands during aging, using mouse models. By comparing the transcriptomic profiles of normal mice to those of EDA mutant tabby mice, which lack sweat glands, we identified 47 genes encoding core secretory proteins in mouse sweat glands, most of which had not been previously identified in sweat glands.
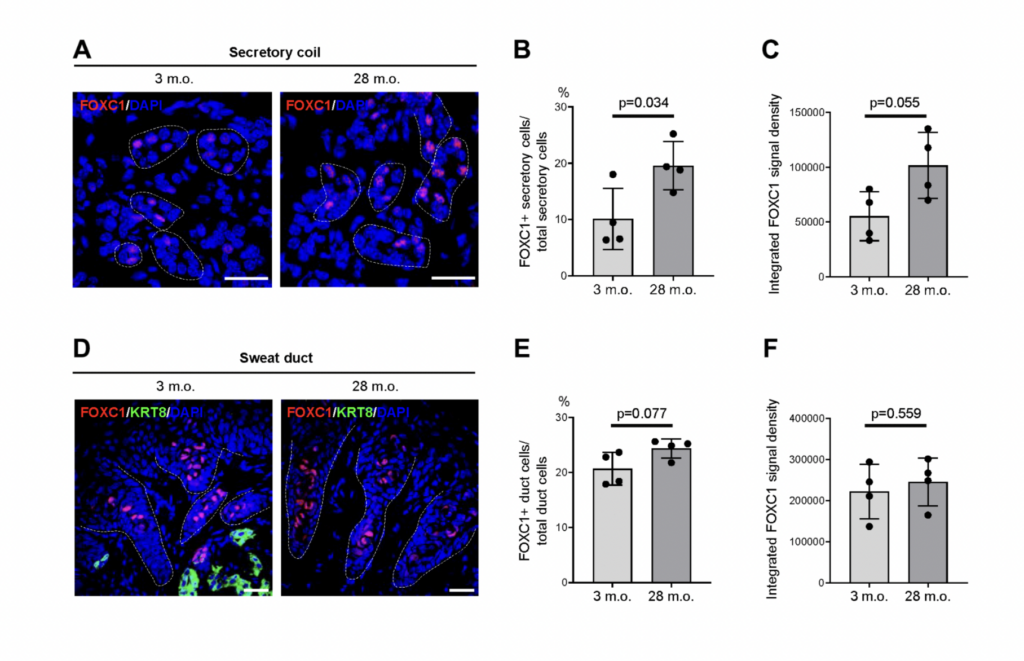
By immunostaining, we validated the sweat gland-specific localization of several of these proteins encoded by the core secretory genes, including FOXA1, BEST2, FOXC1, ATP6V1B1, CIRCA-A3, and VAMP1. Subsequently, we analyzed the expression changes with age of these core secretory mRNAs. We identified 11 of the 47 core secretory mRNAs encoding transcription factors, ion channels, ion transporters, and trans-synoptic signaling proteins that showed altered abundance with age.
Notably, in aged secretory cells in the sweat gland, we found that expression levels of FOXA1 and BEST2, known master regulators of sweat secretion were reduced. While levels of FOXC1 and known transcription factor were elevated. We validated these findings with quantitative RT-PCR, as well as immunostaining analysis.
We hypothesized that these 11 mRNAs differentially abundant in old sweat glands encode proteins that contribute to the decline in sweating capacity observed in older mice.
So this study allowed us to identify genes encoding core secretory proteins and their expression changes during sweat gland aging in mice. Many questions remain to be answered in the future. Firstly, we needed to clarify the function of each of the core secretory proteins that changed during sweat gland aging. Secondly, we needed to analyze the upstream regulatory mechanisms responsible for differential expression of the core secretory proteins. Finally, since we found these core proteins in mice, we needed to determine if the same or similar proteins are affected in aging human sweat glands.
So this study was supported by the intramural research program of the National Institute on Aging. We extend our appreciation to Dr. Myriam Gorospe, Chief of Lab of Genetics and Genomics, for her vital support. We also thank all co-authors of this paper for their indispensable contributions.
Click here to read the full study published by Aging (Aging-US).
—
Aging (Aging-US) is an open-access journal that publishes research papers bi-monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].