In its second season, the Longevity & Aging Series is a video series that features esteemed researchers discussing the latest in aging research with a focus on their studies published by Aging.
—
Below is a transcription of the the second episode of Season 2 of the Longevity & Aging Series. Here, Dr. Ming Yu and Namita Hattangady from the Fred Hutchinson Cancer Center in Seattle, join host Dr. Evgeniy Galimov to discuss a research paper they co-authored that was published as the cover for Volume 16, Issue 4 of Aging (Aging-US), entitled, “Mapping the core senescence phenotype of primary human colon fibroblasts.”
So with me today are doctors Namita Hattangady and Ming Yu from Fred Hutchinson Cancer Center, who focus on gastrointestinal cancers. Namita is a postdoctoral research fellow from Grady Lab with 14 plus years experience of translational research and tumor biology. And Ming is a principle scientist with 15 plus years of expertise in translational cancer research and biomarker discovery, as well as API of NCI-funded research program on translational epigenomics. Namita and Ming have recently published a paper titled, “Mapping the core senescene phenotype of primary human colon fibroblasts,” which they will tell us about soon.
Namita and Ming, please introduce yourself and tell us more about your background and your research.
Ming Yu
Thank you. Hi, thank you so much for this great opportunity for us to introduce our research. So my name is Ming, I’m a principal staff scientist at Fred Hutchinson Cancer Center in Seattle, USA. And I’m really grateful for you and for the editor for giving us this opportunity.
So next I’ll let Namita introduce herself and then she’ll start the presentation. Just briefly go through our published study. So go ahead, Namita.
Namita Hattangady
Thank you, Ming. Yeah, as you mentioned, I’m Namita Hattangady, I’m a postdoctoral research fellow here in the Grady’s Lab and I work under the mentorship of Bill Grady and Ming over here.
I have been mostly working on colon cancer and looking at how the aging process leads to predisposition of cancer in the colon tissue. So with that I can start presenting our work and our
Thank you again. I really appreciate the opportunity that, Aging, the editors have given us to present and talk about our work. Well, it’s certainly very exciting. so the research that we will be presenting today is, was published in the Aging Journal past February.
It is titled “Mapping the core senescence phenotype of primary human colon fibroblasts.” And the first author here and Ming is one of the senior authors, who’s mentorship I worked under. So I’ll just start off with a very brief background. So colorectal cancer or CSC is one of the most lethal and common forms of cancer affecting both men and women.
It is mostly considered age associated, and its incidence is mostly at around 65 years of age. but more recently, there has been a spike in incidence in a younger population as well. A lot has been studied about the mechanisms driving colon cancer and the most commonly known mechanism is the normal to CRC sequence, where the normal epithelium develops a mutation in an EPC gene rendering it hyperproliferative. And then over time, as there is an accumulation of mutations gene rendering it hyperproliferative. And then over time, as there is an accumulation of mutations and epigenetic changes, it forms adenomas or it has the ability to form adenoma and carcinomas. Yet only 5% of these adenomas actually progress to forming cancer. And the entire recent part is not completely known and it indicates a rule for the surrounding tissue microenvironment during this process.
In terms of risk factors, by and large, the greatest risk factor for colon cancer is age and age associated changes. And so we looked at candidate mechanisms that play a role in aging and age associated pathologies. And as you can see here, there are several changes that occur both at the molecular and biological levels, including macromolecular damage, epigenetic changes, loss of stem cells, changes in metabolism, etc. but what we are more interested in is information on senescence.
So what is senescence? Well, senescence is basically an arrest in cell proliferation, but these cells are still very much alive and metabolically active. So a senescence cell is characterized first of all by inability to proliferate, it enlarges in size, there’s an increase in a cup signaling a whole lot of DNA damage. Most popularly or reliably, senescence is identified by an increase in senescence-associated beta-gal staining that occurs at pH 6.0.
The interesting thing about these senescence cells is that when I say their metabolic activity is still alive, they really pump out a whole lot of senescence-associated secretory phenotype or SASP. That is a whole bunch of chemokines, cytokines and growth factors that really get dumped into the surrounding microenvironment. And then these chemokines and cytokines have the ability to go around and modulate the surrounding tissue.
So they really have the ability to modulate the mechanisms of cellular activity and the phenotypes in the local surroundings.
Senescence cells play a role not only in aging but also in normal physiology, not just pathologies in normal physiology. When you think about senescence cells, they are very transient. They get cleared up pretty soon. And so any SASP that they secrete has a very brief and transient effect and it plays a role in development and wound healing and regeneration.
In fact, it even is known to have some tumor suppressor effects. However, with age, these senescence cells keep on building up. So there’s a large number of senescence cells which do not get cleared out because now with age, the senescence cells, the mechanisms for clearing the senescence cells are down regulated and the senescence cells also have a lot of don’t signals that help them to avoid clearance by the immune system.
So now what we have is a persistent SASP just constantly present in an environment and having more detrimental effects. So here we are talking about the changes that are associated with age-related diseases, chronic inflammation and tumor progression. So in the large picture of things, this is a really important facet to understand, because understanding what SASP is secreted from the senescence cells allows us to figure out therapeutic strategies that will allow us to remove these senescence cells and improve overall health span, elongate lifespan. But an interesting fact about senescence is that it has extremely heterogeneous senescence phenotype changes depending on the cellular studying the tissue it originates from and also the stressor that is used to induce senescence.
Our first question was, well do we see senescence cells in the colon? And to address that, we used FFPE tissues from the normal colons of subjects with absolutely no concurrent adenomas or carcinomas. So these are healthy subjects and also the normal colons from subjects with concurrent advanced adenomas.I’d like to introduce a term called, primed colon. We believe that these normal colons here are primed. That is, they already have a very rich environment that is conducive for cancer formation.
So when we looked at these FFPE tissues, we basically looked for stromal cells that are positively stained for phosphogamma H2AX. These are histone modifications seen here in green that occur with DNA strand breaks that occur in senescence. And then we negatively selected for Ki67 because here you can have DNA strand breaks as part of proliferation and that is of course a nuclear counterstain. And then after quantifying all this data, what we found was that indeed subjects with primed colons, that is subjects with concurrent polyps, adenomas or colorectal cancer, have a buildup or an abundance of senescence cells in the stroma as compared to the therapy subjects.
I also want to point out here the amount of heterogeneity in the abundance of the senescence cells as well. And then finally we also look for an association of the senescence cell abundance with H and we found that in healthy tissue seen here in blue and also in normal tissue from primed like the primed colon, the normal tissue from subjects with concurrent polyps, adenomas or cancer seen in red, we also have an association of senescence cells with H. And again, in gray here, these are just all the samples put together. And again, we have significance in terms of association with percentage of senescence cells with H.
Our overall hypothesis again is that the persistence of the senescent fibroblasts and their SASP, creates small pockets or micro-nesias that become conducive for cancer initiation and progression.
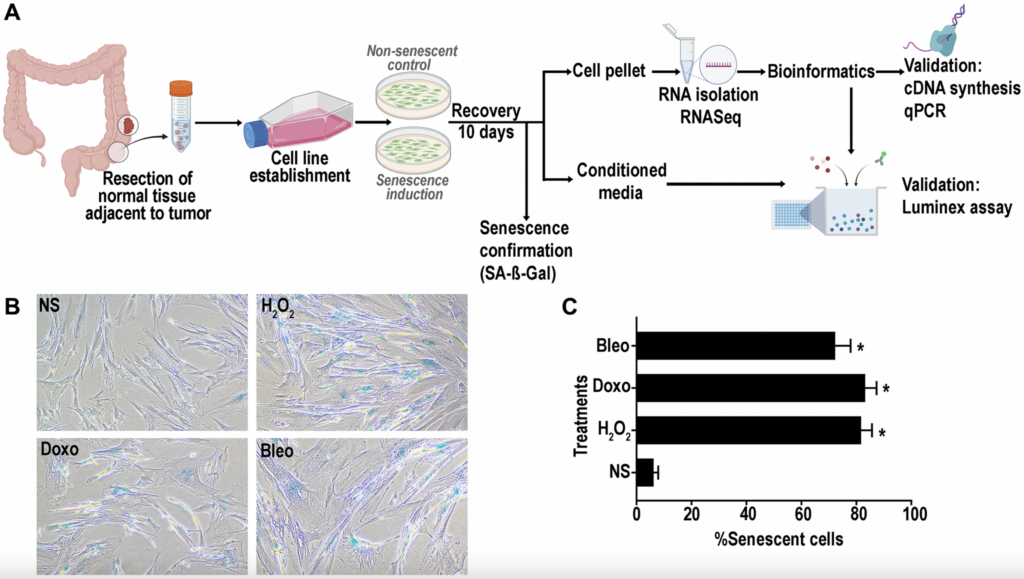
So the next question then is, well, what does the senescence phenotype of colon fibroblasts look like? There are several databases that have data on phenotypes, senescence phenotypes of different types of cells, but it’s not there for colon cells, colon fibroblasts specifically. So that was our main goal for this study. So to approach this, we basically generated, we generated cell lines from normal tissue resected from colons that had concurrent cancer. And using these normal colons, we then dissociated it, generated a primary cell line, expanded it, and then distributed them in four subsets. One was the non-senescent control and then we had three subsets within the senescence induction where we used three different methods of senescence induction. After recovery for 10 days, we then performed the beta-gal assay that I mentioned earlier. And as you can see, our three methods of induction oxidative stress by H2O2 and then doxorubicin and gluomycin, which are genotoxic stressors, increased beta-gal, senescence associated beta-gal activity as compared to our non-senescent cells. And this quantified to approximately 70-80% of our cells. We had pretty good senescence induction.
Once having conformed senescence, we then collected the pellet for RNA isolation and RNA-seq and also for downstream validation by qPCR. And alongside with that, we did also collect the condition media, which was then used for validation studies using the Luminex assay.
So coming to our results now, based on our RNA-seq analysis, we found that – and Ting and Brett here were our bioinformatics specialists – so based on the RNA-seq results, we found that each of these senescence inducers had a unique senescence phenotype. We focused mainly on these red dots here, which are upregulated genes because that’s what we are interested in, in terms of senescence. And then we also created a Venn diagram to identify those transcripts that are upregulated regardless of the type of inducers of senescence.
This common group of transcripts we refer to as core senescence profile. Now within this core senescence profile, we then looked specifically at the subset of transcripts that code for secreted proteins because these are the ones that will actually spread into the microenvironment and have the ability to modulate processes. For further validation from the secreted transcripts, we then looked at or shortlisted eight different, eight different transcripts or candidates based on information that was available about the role they play in different types of cancer.
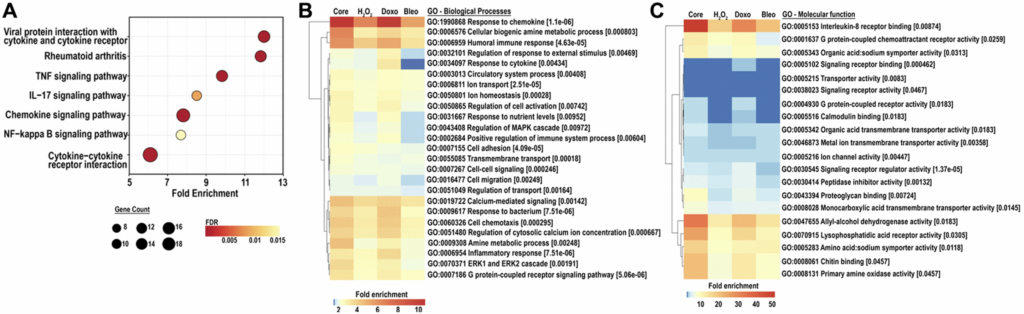
So this is a heat map over here and what you see is basically the color intensity and increase in color intensity shows an increase in gene expression in three different senescence induction methods compared to non-senescent cells. And the data are from three independent subject derived cell lines. So we then perform some downstream analysis, validation analysis, and this is first of all a qPCR looking at gene expression. Here you can see that six of our transcripts were successfully validated as being increased in senescence. Again, you can see there is variation depending on the type of inducer that is used, and also depending on the subject derived cell line as well. Two of the transcripts were not detected because they were below detection levels.
We then looked at validation by of protein using the condition media and again, we were able to validate six of our candidates. You can similarly see heterogeneity based on the senescence induction method and also inter-subject variability. So having done this, we then did a bioinformatics analysis to see what kind of pathways get enriched in senescence, and here are the results. What we see here is the largest pathway that is upregulated are cytokine and chemokine receptor interactions. Besides that, we also have the NFPAPRB pathway, the TNF signaling pathway, both of which are very commonly upregulated in aging and age-associated pathologies. Finally, interestingly, we also found an IL-XV signaling pathway that is upregulated indicating a potential change in some immune mechanisms as well.
We then moved on to perform a gene ontology analysis. Here we look at biological processes and this is the long list, so you don’t need to read through everything. But what I really want to point out are just the response to chemokine, the activation of various cells. This list removes a lot of redundancies, but when we talk about cell in terms of activation, migration and chemotaxis, it involves neutrophils, leukocytes, and a lot of different myeloid derived cells as well. It really shows us the potential pyroprane effects that these SASP candidates have on the microenvironment. When you look at molecular function, the topmost is interleukin-8 receptor binding, interleukin-8 or CXCL8, was one of our core SASP candidates that we validated. There are a lot of other molecular mechanisms that are also modulated in this process.
In summary here, we described four senescent profile for primary human colon fibroblasts. For the very first time, we described several candidates that were validated at both the transcriptomic level by qPCR as well as at the Luminex assay. We showed from bioinformatics analysis that the NF-PAPRV, the TNF pathways both well reported in aging and senescence are also enriched in our senescent cells in vitro. Finally, that our bioinformatics analysis also identified an enrichment inside of chemokine-related mechanisms and the activation of various types of immune cells.
So with this, I’ll stop my presentation and we are very happy to discuss anything related to the data we presented here, but I cannot stop it before presenting an acknowledgement slide because I’m so appreciative of the entire team, Bill, Ming and all our lab mates and all our funding sources and collaborators both at the Hutch,nationally and internationally. So, thank you.
Evgeniy Galimov
Thank you very much. Great presentation.I would like to ask first, how can we use these discoveries? How can core phenotype be used in clinical practice and in fundamental research as well?
Namita Hattangady
That’s a great question. So right now we’re really looking for senescence-associated chemokines, cytokines and growth factors that play a role in creating this pro-conducer microenvironment and actually leading to progression of cancer.
Of course with further studies, we are very hopeful that if we identify clear drivers of senescence, they can be used as therapeutic targets and also perhaps as a biomarker for identifying progressors versus non-progressors. So it really helps us with a more personalized approach to treating cancer.
Ming Yu
I also like to maybe add on what Namita just said. Again, great presentation, Namita. One thing I’d like to point out is as Namita pointed out, colon cancer is age-related sort of age-related disease like other cancers or other diseases. We think that maybe this senescence plays a role in driving the cancer. So we think maybe those anti-aging or anti-senescence therapeutics actually has a way to be more cancer-preventive, especially for the older generations. So again, this is something we are currently exploring in the lab using the pre-clinical models, but we think anti-aging therapeutics actually has cancer-preventive, especially in the case of colon cancer.
Like Namita pointed out, we not only want people to live longer, but we also want people to live healthier and hopefully cancer-free. So that’s our ultimate goal.
Evgeniy Galimov
Thank you. That’s quite detailed. I want to touch on this biomarker side. Is it possible to somehow measure the presence of SASP and colon? What will be your approach to this once you find the factors relevant?
Namita Hattangady
So we been actually, Ming has been spearheading this project and I’ve been working along with her, but we have been looking at senescence in colon tissue, particularly in early and late adenomas using spatial transcriptomics. These are still very early studies, but we have identified the presence of several SASP candidates in early and late adenomas.
Of course, we’ve also performed other studies such as using QPCR. We have already identified several SASP candidates IL6, IL8, and MMP-III, MMP-I, many of them using simple analysis like a QPCR from using FFPE tissue. We’ve already done some analysis there. But now with more advanced technology, we can actually have better resolution using methods like 10x Visium or GeoMX.
I’ll let Ming talk more about this because she’s really spearheading this project, but we do have a couple of targets that look pretty promising and are currently getting prepared for publication.
Ming Yu
Namita already touched on a lot of the key things that we are doing in the lab. But what I to point out is our lab is also one of the participating labs in the national wide early detection research network. So we already have this biomarker discovery and validation pipeline infrastructure set up in the lab. And it’s just a matter of how we can identify those prominent SASP factors and we can use our infrastructure to fasten the whole discovery workflow. But one thing I’d like to point out is the GDF15, like we identified – Namita also showed nicely about the validation – GDF15 is very interesting in the past we already published study a few years ago mainly elucidating the biological role of GDF15 in driving the, as a SASP factor, creating this micro environment to promote cancer initiation and progression in the colon.
But now we’re interested in actually looking at GDF15 in the serum patients, especially for those patients that we think have a high risk of developing cancer. So we are again, using our infrastructure from EDRN and then we are trying to see if we can identify, use GDF15 as a biomarker to identify those high risk patients and also we can maybe think about doing some preventive approaches. So this is something again, we are actively pursuing in the lab.
Evgeniy Galimov
Thank you. I’m also curious about the relationship between senescence and colorectal carcinoma. As you showed on the slides, there are some associations. So can you really predict, do you have any predictive models for colorectal carcinoma based on any biomarker available at the moment or its future only?
Namita Hattangady
So like the slides showed, we definitely see an association of senescence burden with age of subjects overall in a broad spectrum. But I would like to add also, that we do need to keep in mind that there are a lot of confounding factors to keep in mind as well for each of the subjects.
Right now, we have a relatively small human data cohort. We need to expand that cohort to ensure a lot of representation of different races, ethnicities, genders, and to do any kind of predictive analysis of that sort. Because you can see the heterogeneity even in the senescence burden. So you’d need a lot of subjects which are almost outliers in terms of senescence load to be able to create that really nice discovery subset and then a very good validation subset to be able to then go ahead and do any kind of predictive analysis.
Ming has been spearheading a lot of parallel projects, so many parallel projects and some in vivo with nice models and I’ll let Ming continue and talk about her studies.
Ming Yu
I think that there must, be we already have experience or we have expertise in this field looking at how we can translate our findings in the lab and then maybe either build a predictive model so we can actually identify those targets and then develop assays and do more clinical validation.
Again, GDF15 is one of the things that we are pursuing right now, but on the other hand, we also think it’s also really important to elucidate a lot of the biological functions of the SASP factors before we can definitely say senescence really is driving the cancer initiation and the progression in the colon.
Evgeniy Galimov
Thank you. I noticed in the paper you mentioned that in the gut normally there are lots of genotoxic stressors which basically can induce senescence and late colorectal carcinoma.
Can you recommend anything about food or dietary habits which could decrease genotoxic stress in our gut?
Namita Hattangady
I think the most common sources of genotoxic stress rate from the gut would probably be red meats and processed foods. Then of course there’s also microbiota and there’s so many different things that control the microbiota and the gut, including your source of water, how clean your foods are and so on and so forth, that finally impact gut dysbiosis and the toxins that those different microbiota need. But in terms of food habits, of course less processed foods, more fresh foods, eating lots of antioxidants and of course not smoking, not drinking, a healthy lifestyle. I think those are the common things that I can think of.
There are several other factors where you’re geographically placed and what race you belong to. We know that the African American race is more susceptible to colon cancer, particularly men. So there are several other factors to keep in mind, but in terms of living healthy, just eat well, eat healthy, don’t eat processed foods and meats and exercise.
Ming Yu
So that’s a good question, so I’d like to also add a few points here. First is, we also have a study already close to publication. This is a collaboration with gut microbiologist/physician at the Hutch, and his name is Neel Day. We have this collaboration where he is using the motor biotic mice model, a mouse model, which is he can actually give the mice the different microbiome in the mouse model of colon cancer. And also he actually identified again, the SASP factors plays a role even in the microbiome studies of this colon cancer. So really this is a study that we are about to publish, but I also want to mention is this genotoxic factor or the stress, those are from external.
One thing to keep in mind is really the reason why the senescent cells accumulate is actually probably due to the fact that as people grow older, their immune system is getting weaker. So in the young people, normally the senescent cell, Namita made the point about they’ll not be hanging around. So immune cells are very efficiently cleared up. However, as we grow older or as we age, the immune system starts to not be able to clear up the immune cells, clear up the senescent cells and so they accumulate the dumping lot of SASP in the tissue microenvironment and then that’s where the problem arises.
When we talk about how we can use this mechanism to change the lifestyle, we also need to think about how we can boost our immune system, especially in the aging population. And so it’s external and internal factors, they both work together to make sure our system is clean and we can remove all those zombie cells or we call senescence zombie cells because they should be dying but they’re still hanging around. Just make sure our body is just optimal. Even when we go into the older or we grow older. So that’s the whole idea of the in and out approach.
Evgeniy Galimov
This core SASP phenotype. What could be the commonalities between colon core SASP phenotype and other tissues core phenotypes? Can you add anything about that?
Namita Hattangady
That’s a great question. We actually did compare our core phenotype to the core phenotypes that have been published previously. I think one of the biggest datasets that we looked at was with analysis of lung fibroblast on epithelial cells that were rendered senescent using different methods just like we did. When we compared our core colon fibroblast phenotype to their core phenotype of pulmonary fibroblasts, we found a pretty large overlap in a lot of genes. Many of them were genes that we have already validated like CXCLE then GDF15. We also compared our core SASP to what they call a universal senescence profile.
These are commonly up-regulated genes and senescent fibroblasts and epithelial cells from the lung. And we, again, found that GDF15 that Ming was mentioning, it was, upregulated common to all our systems. Then, we also did treatment specific comparison. Our doxorubicin-induced senescence phenotype to another study where they used doxorubicin-induced senescence in pulmonary fibroblast, and there was a 61% overlap, a really large number of genes. Again, a lot of them were part of our core phenotype GDF15, and CXCLE, a CCL20 as well.
And then, interestingly, we also compared to the aging secretal, and found a lot of overlap there as well. This was mainly for physiological relevance. We saw a lot of aging related CP3 candidates as well. Finally, there was a very recent study published by the MIR group, and they published a set up list, which was beautifully curated. These genes are commonly identified in senescence and aging across mice and humans. There was almost an overlap of around 25 to 30 transcripts. Again, when you consider a core of core phenotype common across tissues, across cell types, I think one of the main things that came across was GDF15, along with a few others.
Now, of course this list will change depending on our stringency criteria. We use very, very stringent criteria. We used FDR 0.01, and a whole change of a minimum of two. But really, were very, very highly upregulated genes. Certainly, this will increase depending on what criteria we use. GDF15 and a few others come up as really significantly. CXCL and GDF15 significantly upregulated in senescence across cell types and senescence inducers.
Evgeniy Galimov
Great. But just want to double check about this aging secretome. When you say aging secretome, do you mean some kind of database about several tissues senescent SASP…
Namita Hattangady
…no, this is using serum from subjects of different ages. And then, they did basically a study looking at which of the different proteins that they identified in the serum are actually associated with each.
Evgeniy Galimov
Okay, great. Ming, do you want to add something?
Ming Yu
Again, the aging secretome, we are very delightedly surprised to see there are a good amount of overlap between that. Very different study that, again, speaks back to your question about whether we can take our list of core SASP and then think about whether some of them are actually we can detect them in the serum, and then think about how we can develop a biomarker assay to either monitor the cancer risk in the population, or we can actually use that as more like early detection. So those are the things that we are actively pursuing in the lab.
Evgeniy Galimov
I’m also curious about cancer senescence. Cancer cells can also become senescent after treatment. Do you think they’ll become analogous in the course senescence cells phenotype between cancer senescence and normal colon cell senescence?
Namita Hattangady
I think, yes. By the design for our experiment, I think you will see commonalities because the genotoxic stressors that we use are actually chemotherapeutic agents. And as we talked about, both those inducers lead to SASP candidates that are upregulated in different types of tissues. I would think that there will be some overlap with therapy induced senescence as well.
Evgeniy Galimov
I’m also curious about the treatment. You mentioned in the paper that targeting enough kappaB pathway can be really used to decrease point inflammatory of SASP component. But I’m curious about, is it known anything about targeting TNF-α, part of SASP? Or also, using senolytics in general apart from xenomorphic effects?
Namita Hattangady
I’ll let Ming answer this because she has actually been leading efforts on senolytics and in xenomorphics in the lab.
Ming Yu
I’m happy to share. First is, again, based on hypothesis, we think senescence actually, or cellular senescence is one of the drivers, at least in the colon cancer setting. Again, that’s the direction we are always going, so whether we can use anti-senescence therapeutics for cancer prevention, or even for cancer treatment. In terms of the current agent therapeutics out there, as you mentioned, there are mainly two different types.
One is called xenomorphic, and those type of treatments really make the senescent in check. As you mentioned, there are the MF kappaB targeting agents like metformin, but in the lab we actually are using M1, C1-inhibitors called rapamycin. And again, rapamycin or metformin has been shown to have an anti-aging effect, but now we are really testing if those also have anti-cancer effects. We are currently using the mouse models of colon cancer and treating them with rapamycin, and then just see if the treatment can reduce the tumor load in the preclinical models.
The second type of therapeutics we are pursuing is called senolytic, mainly just to clear up the senescent cells. Those are represented by the drug called DnQ. We call it Dasatinib and Quercetin. Again, that’s also one thing, one experiment that we are also in the lab pursuing is to treat the mouse models for colon cancer and then treat them with the DnQ. We call it DnQ. And again, check the hypothesis if we can. By clearing up the senescent cells, we actually, again, can reduce the tumor incidents in the mouse model before we actually can safely say that those drugs have this anti-cancer effect.
We are hopeful, but we’re very cautious. Not until we have very exciting promising data from the preclinical, then we can for sure conclude anything that anti-senescent drugs actually can prevent cancer or have this anti-cancer effect.
Namita Hattangady
And also, to quickly chime in here, even besides cancer, just in normal mice and also some human trials there have been studies showing that treatment with DnQ, this treatment is given sporadically once in a few months. And that actually very much improves overall lifespan and healthspan in mice, and some of the results have been seen also in humans in early clinical trials. It is very promising, but like Ming said, in the case specific case of colon cancer, until we get our data and see the data in our hands, we’d have to see how it comes out before we make comments.
Evgeniy Galimov
It’s amazing you already are trying to understand the effects of senomorphics and senolytics in clinical practice. It’s amazing.
I also would like to ask about, I see your focus in the paper on the mostly pro-inflammatory pro-tumorigenic SASP components. But as you said, transient SASP can actually be anti-tumorigenic, aiming to clear tumor cells. Is it possible, or do we have any core phenotype or any proteins which are anti-tumorigenic? And can we really induce this or any other drugs?
Namita Hattangady
Would you like to take a stab?
Ming Yu
I think here is, like Namita mentioned in the earlier slides, transient senescence or cellular senescence is actually beneficial. But I think what we are worrying about is this persistent senescence hanging around in the tissue and then causing damage. That’s how we design our study to really see whether those persistent phenotypes, or SASP phenotypes, have this microenvironment modulating effect, and how those attract immune cells and to create this pro-inflammatory response in the tissue.
Then assay also leads to the cancer initiation, especially in the colon. But in terms of those transient senescent phenotypes, again, I think one thing to keep in mind is a lot of those, like the chemokines and cytokines, that also – some of them validated by Namita – but there were also other things that we haven’t discussed or we haven’t shown that Namita is actually pursuing. One of them is called PAPP-A. PAPP-A is something like – Namita will talk more about this – but we think PAPP-A is also one of very interesting SASP factors. And again, maybe Namita, you can talk more about this. Because that’s one of the study that Namita is currently leading, and we are close to publication.

Namita Hattangady
Sure. PAPP-A is an enzyme, it’s a metallic proteinase, zinc metallic proteinase. And it’s basically an enzyme that targets IGF binding proteins. In neurophysiology free floating IGF1 is kept in check by binding to IGF1 binding proteins. PAPP-A is one of the predominant enzymes that can break down the IGF binding proteins and then release IGF1.
So now IGF1 is available to bind to receptors in that local environment, and only to perpetuate downstream effects of IGF1, many of which are, proliferation and also invasion, metastasis and so on and so forth. So we found that, PAPP-A, in fact, is very rich and active. The network is very active in early adenomas and specifically – this is based on spatial transcriptomics – specifically in more epithelial cells that you can find so very close to a epithelial cells. so we looked at what PAPP-A goes to, at the little cells, if it is present when it is bound to IGF1. And we found that specifically in the cells, we found that IGF1 gets released when PAPP-A breaks down. It then binds the IGF1 receptor – so we’ve seen also poor activation downstream signaling mainly through the PC. And we’ve seen an increase in proliferation and is an invasion. So we barely seen these poaching or genic effects very specific to adenoma cells in the few cancer cell lines that we’ve looked at. We really do not see those effects. Of course, we’ve not looked at the entire plethora of cancer cell lines available out there. But in the ones that we did check, PAPP-A did not have as large a role as it did in adenoma. It’s one of the first studies or first candidates that we found that is almost like adenoma-enriched mechanism.
Ming Yu
Another thing I’d like to also add on is, as you mentioned about this anti-tumorigenic versus pro-tumorigenic effects by cellular senescence. And here I’d like to point out is, depending on the cell type, like what kind of cell undergo senescence. For example, there is a specific phenomena called oncogene-induced senescence, and occurs actually in the epithelial cells. When it does happen, like KRAS is a strong potent … with KRAS mutation, again, this strong potent inducer of this oncogene-induced senescence. And when it occurs in the colon epithelial cells, it actually induces senescent phenotype in the epithelial cells and acting more like a stop checkpoint for the cells to stop perforating and then before the cells become neoplastic. Again, if this happens in epithelial cells, if the senescence occurs in epithelial cells, it works as a break, as a checkpoint to tell the cells stop proliferating and do not go becoming cancer. In this way, cellular senescence in epithelial cells actually act as an anti-tumorigenic additional proof to make sure things are not going bad down the road.
Namita Hattangady
The other thing that I would like to add in here is that, yes, you have these antitumor effects, and when you have these pro-tumorigenic effects. But really, what you’re looking at is the whole picture. The SASP cocktail as a whole from these different cell types. A lot of SASP candidates, including CXCLA, CXCLR, CXCL20, for example, have the ability to go into the micro environment and then actually reinforce senescence, and also perpetuate the senescence. They bind the Cxcr2 receptors and activate NF-kappaB pathway in other cells and also in their own cells fashion. So, what you end up having is this overall senescent tissue environment, or an inflamed environment. So you have a battle of antitumor and protumor, and finally, what determines things are various factors, including the presence of immune cells there. Which immune cells? Are they T cells or are they more functional T cells? Is there exhaustion of cells happening?
There’s so many factors that they enroll, that really we need to look at an overall picture. And that’s where those spatial transcriptomic studies are directed towards that Ming is spearheading.
Evgeniy Galimov
Thank you. In talking about the induction of senescent cells, initially. So I see in the paper you talk about, stress induced senescence, oxidative stress and toxic stress. Are there any other factors or stressors? These, causes of, senescence, induction of the gut?
Namita Hattangady
Overall, aside from the gut, of course there are lots of different senescence stressors. Even in genotoxic stressors, for example, gamma radiation, X-rays, UV rays. UV rays are so common in causing skin cancer, which is why we put so much sunscreen on. But specific to the colon, of course, we have so many microbiota, we have the genetic stress that is happening. We have different producers of oxidative stress, for example, ER or mitochondrial dysfunction. There are definitely a lot of different mechanisms that are happening together at the same time. But since we were doing it in experiments in a more reductionist form in our lab, we just used what we thought was the most common methods that accumulate with age. So we ended up using these specific agents for senescence induction.
Evgeniy Galimov
Lastly, I would like to talk about the limitations of the study. You mentioned that there are lots of heterogeneity in SASP in the cells. Can you tell a bit more about the causes of this at the hydrogenation?
Ming Yu
Yeah, sure. I can start, and Namita can chime in. Yeah, like you pointed out, I think one of the more limitations of our study is we did see this heterogeneous response to the specific senescence inducer treatment. That’s one thing. The second thing is we also observed this heterogeneous senescence load when we … If you can remember, we showed that figure showing the percent of senescence cells we defined as damage to X positive, K as seven negative. We also see a five spread. And I think I’ve seen more of the common challenges of working with human samples. And this heterogeneous response, or more like the variation in percent of senescence load from patients I think really reflects more like the heterogeneous human population in terms of subject’s age, gender, and other demographic difference.
But gladly, I feel like we still are able to see a very strong and statistically significant difference when we look at either their response to the specific SASP senescence inducers, or when we compare the subjects from cancer and then compare that from subjects from normal, with normal colon. I think really, although we do see heterogeneous responses, we still are able to present statistically significant results in our SASP edification and validation study.
I think that’s one limitation, but at the same time we feel like our data is also pretty solid and strong.
Namita Hattangady
I have nothing more to add. I think Ming touched on all the points.
Evgeniy Galimov
I found in limitations that there was only one time point where you measured senescent SASP. Can you hypothesize about temporal changes? Can you tell a little bit about that?
Ming Yu
I can start and Namita can chime in. Like you pointed out, really the SASP that we presented in our paper, it is from one time point. But there was this study that we did in the lab where we didn’t publish this in the paper, is we did check for some prominent SASP factors one month post senescence and then three months post senescence. And we really see this increase, actually, in the sum of the representative SASP factors in terms of gene suppression over time after the cells undergo senescence.
We just think about in vivo setting is if a senescent cell heading around right enough, the longer they are in the body after the more SASP factor they’re going to produce and they’re going to accumulate in the microenvironment, and that the more damage, the more inflammatory response the SASP will elicit. And then that’s where the thing’s going to go wrong, leading to cancer, leading to a lot of inflammations, leading to a lot of age-related pathological changes in the body. But again, that’s something we do like to point out is really the degree of senescence secretion, SASP secretion, and the more like the detrimental effect that’s related to how long the senescent cells are around in the body.
Namita Hattangady
And just to add on to that, besides the SASP factors that we looked at, we also looked at another molecule called HLA-E, which factors don’t need a ‘don’t eat me’ signal. That’s a molecule that helps the cell to evade clearance. That’s also something that we found increases over time with senescence. So one month, three months and so on, actually it keeps increasing. And this we checked using flow cytometry, so surface marker expression. It’s not exactly secreted that we looked at for a surface marker.
This is what tells us what the mechanism is like, in vivo helps us speculate that as with time that’s how the cell finally manages to evade clearance, keep on staying on, the SASP keeps on building up leading to a toxic and more toxic and more toxic environment, which spreads into the microenvironment and then perpetuates and reinforces it. That’s how those little pockets or niches of tumor conducive environments and such.
Evgeniy Galimov
And maybe the last thing to discuss is, how do you think single cell technology would improve or add to this study, potentially?
Namita Hattangady
I will let Ming answer this, because she has some exciting things to talk about.
Ming Yu
Again, that’s a great question, and that’s also something that we are literally currently doing in the lab is to use single cell RNA 6. And to, again, further elucidate the gene … First of all, what are the cell populations present in the body, especially in the colon? And second of all, again, what are the gene signatures that are producing by these cells? The reason I like to talk about cell type first is although our published paper here is focusing on the colon fibroblast, but I think based on previous studies there are also other cells undergoing senescence, especially the immune cells. Which is surprising, because we thought… the one cell type I’d like to point is neutrophils. We thought neutrophils are very short-lived, but from our preliminary data we actually found the senescence signature in the neutrophils in the body, especially in aging body.
We are very curious about that, and that’s why we would like to carry out the single cell RNA 6 experiments. First in the mouse models, and then we want to extend that into human, and really have a full picture of, what are the cell types that can be senescent? And then, again, what are the skin signatures produced by these cells?
Also, of course as you see, the next logical step was you see, whether indeed anti-senescent therapeutics would have more effect than we originally thought. Those are the thinking, and those are the studies that we are undergoing right now.
Namita Hattangady
And just to add on or to give us an example, we’ve also looked at individual SASP candidates in publicly available data. For example, that we mentioned in pregnancy associated plasma protein … this is an enzyme that we looked at in publicly available data, and we found that PAPP-A is in fact very enriched in fibroblasts. Now we know where the source is from. Now, it’s also present in other cells, but it’s mainly from fibroblasts. And if you compare normal colon versus a colon adenoma, earlier adenoma, versus advanced adenoma, you can actually see that the early and advanced adenomas have higher fibroblast-associated PAPP-A as compared to normal tissue … fibroblast.
Even beyond just looking at the entire senescence phenotype, just in looking at individual SASP candidates, I think single cell data provides a lot of information. And the efforts that Ming is leading, it’ll give us even more information into how pathogenesis actually offers an initiation and progression in cancer and its relation to senescence.
Evgeniy Galimov
Great. Great to see exciting new avenues. Namita, Ming, thank you very much for your time, and it was very nice to talk to you. And all the best.
Ming Yu
Thank you so much. Yeah, we really appreciate that, it’s a great opportunity for us to talk about our research and then talk about all the other exciting things happening in the lab, as well as where the next step will be.
Again, we are grateful for this opportunity and we are looking forward to working with you again in the near future when we have our new findings that may be published in the Aging Journal and also in other journals.
Namita Hattangady
I echo Ming’s remarks. Thank you very much for the opportunity.
Evgeniy Galimov
I’m looking forward to discussing your new papers.
Ming Yu
Thank you, and have a great evening over there.
Click here to read the full research paper published in Aging.
—
Aging is an open-access, traditional, peer-reviewed journal that publishes high-impact papers in all fields of aging research. All papers are available to readers (at no cost and free of subscription barriers) in bi-monthly issues at Aging-US.com.
Click here to subscribe to Aging publication updates.
For media inquiries, please contact [email protected].