Below is a transcript of an interview with Dr. Dale Bredesen detailing his research that was published by Aging (Aging-US) on September 21, 2020, entitled, “Alzheimer’s disease as a systems network disorder: chronic stress/dyshomeostasis, innate immunity, and genetics.”
Behind the Study is a series of transcribed videos from researchers elaborating on their recent studies published by Aging (Aging-US). Visit the Aging (Aging-US) YouTube channel for more insights from outstanding authors.
—
Dr. Dale Bredesen
Hi everybody. I’m Dale Bredesen. I’m a professor at UCLA and I have a long-term interest in the neurodegenerative process. My laboratory worked for 30 years in what is the actual fundamental nature of the degenerative process with the idea that if we could get an idea of what was actually causing, what are the molecular species that are actually driving the neurodegenerative process in diseases like Alzheimer’s and Parkinson’s and Lewy body, in frontotemporal dementia, and ALS and on and on and on, then we could begin to fashion the first effective treatments. Because many would argue that this has been the area of greatest biomedical therapeutic failure. As they say, everyone knows a cancer survivor, no one knows an Alzheimer’s survivor. And so this is a, I think a very exciting time for all of us looking at the changes in the way we translate basic research principles.
We’ve published a number of papers in Aging, beginning in 2014 with reversal of cognitive decline a novel therapeutic program. And then more recently ones on additional patients with Alzheimer’s who saw improvement. And then last year in 2020, paper on looking at Alzheimer’s as a systems network disorder. I think that one of the fundamental changes that we’re all seeing is this change from the idea that Alzheimer’s is a simple disease. We just don’t understand what causes it, but it’s something simple. We’re going to write a single prescription and that’s going to cure it at some point and we just haven’t found the prescription yet. And I think that people are beginning to understand that this is a little bit like asking, what single prescription could you write for someone who is in the end state of widely metastatic cancer?
That Alzheimer’s disease is a more complicated illness. Certainly the epidemiologists, the microbiologists now who are showing us different species in the brain, the neuro pathologists, who are showing us the different pathologies, including things, not only plaques and tangles, but also things like Lewy body and TDP 43, and things like that in many of patients with Alzheimer’s, are showing us that this is a relatively complicated illness and that there are many different potential contributors. And that this has, of course, led to many different theories of some people believing that this is just a disease of amyloid. If we get rid of the amyloid, then things will be better. And, of course, that hasn’t worked with multiple drug trials. Then others believing it’s about Tau, others believing it’s Type 3 diabetes, others believing it’s about herpes simplex, and you just go on and on and on down a very long list.
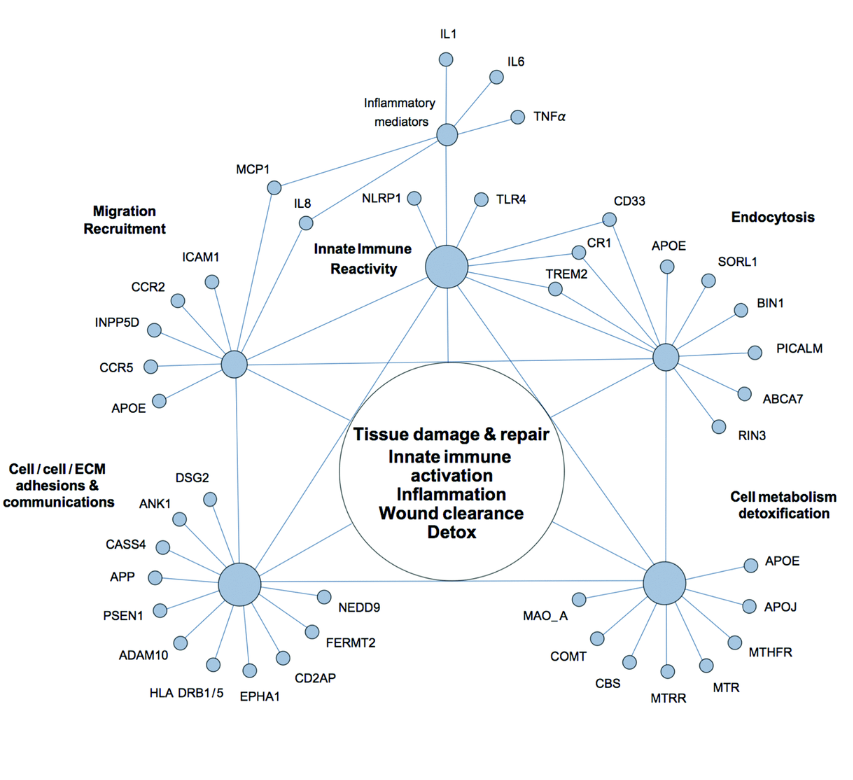
Unfortunately, none of these targeting these single ideas hypotheses has led to any sort of reversal of cognitive decline. So our work suggested to us that we need to look at this as a network disorder, that fundamentally there is a neuro-plasticity network and that other neurodegenerative diseases have other sub systems of the brain. And in all of these cases, you have a supply and a demand. And in these diseases, unfortunately, the supply does not meet the demand in a chronic way or repeated way so that you undergo this downsizing. You’re not able to meet the demands of the system over time. And that for each of these, there seems to be their own unique requirements. In Alzheimer’s disease, there are multiple things that have come back again and again and again through epidemiology and other studies.
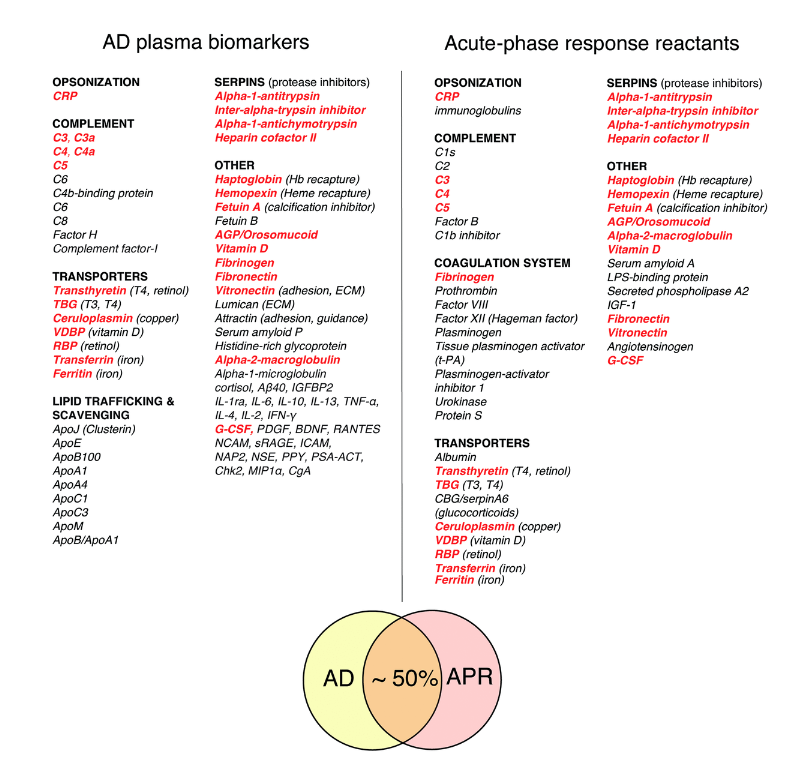
So, anything that activates NF-kappaB, any sort of ongoing inflammatory process, and this could be through things like changes in the oral microbiome, poor dentition, chronic sinusitis, hyperpermeability of the gastrointestinal lining, so-called leaky gut, and on and on, there are many of these, and then things that are any sort of toxicity, and these can be biotoxins, they can be inorganics, they can be organics. Of course, there’s more and more interesting work on air pollution and its relation to increasing risk for cognitive decline. And then energetics, and this includes everything from blood flow to oxygenation. There are so many people who have cognitive decline in association with sleep apnea and nocturnal hypoxemia, and then things like mitochondrial function and even ketosis. Having appropriate ability to burn both ketones and glucose so-called metabolic flexibility.
Then the final of these is around trophic activity. Of course, that’s been known for years. Increases in things like BDNF can reduce risk for cognitive decline, and at the same time reductions in things like nutrients and hormones, sudden reductions in estradiol, testosterone, nutrients, like vitamin D and vitamin B12, all of these are potential contributors. So, with that in mind, I think there’s a huge amount of activity in this whole area where we really had nothing just a few years ago. We’ve recently posted the results of a clinical trial in which 84% of people actually saw an improvement, not simply a slowing of decline, but an actual improvement in their cognition, improve on MoCA scores, improvement in CNS vital signs, and online cognitive tests, improvements in so-called AICCU change scores, where their partners are noticing improvements, and, interestingly, improvements in their MRIs.
Improvements in gray matter volume and less reduction in hippocampus volume, and you see even in normal people, not just people with MCI or Alzheimer’s. So we’re very excited about that. And we’re now in the midst of the planning stages for the next larger trial that will be a randomized controlled trial as opposed to a proof of concept trial. So a lot going on. Just published a book recently called, The First Survivors of Alzheimer’s. These are people going all the way back to 2012, over nine years on the protocol that we first reported in Aging in 2014, who have sustained their improvement, and that’s very important, obviously. Getting people to bump up is one thing, but keeping them improved for many, many years, of course, is the goal that we all have for seeing better outcomes for cognitive decline. Especially since Alzheimer’s disease is something that’s on the rise.
Professor Kristine Yaffe has shown that this is now, if you follow serial autopsies, this is now the third leading cause of death in the United States. It’s actually the number 2 cause in the UK. So an incredibly common problem. Along with that, Dr. Heather Sandison down in San Diego has really changed the paradigm for assisted living. She’s gone from, assisted living being a place where you go to die, you just simply go in and you go downhill, to a place where using a precision medicine sort of protocol, looking at all the different potential contributors to cognitive decline, she’s seeing residents come in, and even though they’re relatively far along when they come into an assisted living, she’s seeing that they either will stabilize or actually improve.
So, the idea of going into a residential assisted living facility and actually improving, and then leaving the facility again is really a fundamental change in the way we think about these assisted living facilities and very, very exciting, I think. So I think that the future in this area is changing dramatically. This is a very exciting time where we’re beginning to see people look at complex chronic illnesses such as Alzheimer’s disease in more and more networks and systems biology sorts of ways. The old fashioned idea of getting a very small data set and then just writing a prescription, I think is being replaced by the idea of getting much larger data sets. This is really about 21st century medicine, and looking at what we can do to correct what is essentially a network disorder. This is a very exciting time to see those changes.
I think we’re going to see more and more people looking at these larger and larger data sets and looking at the critical pieces. One of the big issues that’s come up, of course, is with the COVID-19 pandemic. This has put many more people at risk because this does impact the brain. Many people experienced brain fog during COVID-19. One of the big questions has been whether this will lead to a burst of cognitive decline downstream in the future, and time will tell. But certainly we recommend that anyone who’s had COVID-19, and I’m just getting over the Delta variant myself, get on active prevention, and I got the Delta variant as so many other people did despite full vaccination and despite precautions, etc. So it’s a very contagious virus, and may unfortunately lead to a future where we see more neurodegenerative disease.
There have already been three reports of Parkinson’s in association with COVID-19. So I think that this is certainly a concern for all of us for the future, and again, you think about just the numbers, over 600,000 people in the United States have now died from COVID-19. For comparison, nearly a hundred times that many of the currently living Americans, somewhere around 45 million of currently living Americans will die of Alzheimer’s if we don’t have an effective approach to this. So this is the future, and we believe that everyone who turns 45 or who’s over 45 should be on active prevention. Especially if there’s a family history. One of the big issues that we’re faced with, is that we’ve defined this disease classically as Alzheimer’s disease.
But we now know from the work of many different groups, that this is really an end stage. If you think about Alzheimer’s in four general stages, then it becomes clear that we really need to get in earlier, just as things like pap smears really helped cervical cancer. Certainly things like mammography have helped to reduce deaths from breast cancer. When we look at Alzheimer’s disease, there is a first stage, and it’s very clear, you can see abnormalities on PET scan and spinal fluid, but you’re asymptomatic for some period of time, and then roll into a SCI, subjective cognitive impairment, which really should be called early Alzheimer’s disease. There are clearly abnormalities on PET scans and CSF. People know that there are now symptoms. This may last 10 years, so we really have a tremendous window of opportunity for making people better.
Yet what happens is, people go in and they’re told, “Yeah, you’re still testing in the normal range. There’s nothing to worry about,” and so therefore, we lose an opportunity often to do something that’s really going to reduce the global burden of dementia. So the third out of four stages is what is called mild cognitive impairment, and that really should be called advanced stage Alzheimer’s. This is a little bit like selling someone that they have mildly metastatic cancer. It’s a late stage of the overall process, and if we could get people to come in early SCI or early MCI, then we wouldn’t see people who are at these late stage. So, there’s the fourth of four stages, which we call Alzheimer’s, where you actually have now begun to lose your activities of daily living. This really should be thought of as final stage Alzheimer’s.
This is a very, very late stage of a process that’s typically been ongoing for about 20 years. So, again, the push in cancer has always been toward earlier pickup, earlier evaluation toward earlier reversal, and we need very much to do the same thing with cognitive decline and not wait until we’re actually calling it Alzheimer’s, and this is a very, very late stage of the process. Having said that, of course, we still want to develop effective treatments for people who are even in the late stages. We have seen, anecdotally, some people who have cognitive decline with MoCA scores of zero get some improvements, but they don’t go back to perfect MoCA scores of 30. They go back to five or seven or nine, which they make a lot of subject difference, and they do things like start to speak again and start to dress themselves again.
But these are the exceptions, not the rule. So we really would like to see people getting in earlier and earlier. So, overall, I think that this is a tremendously exciting time for all of us who are interested in best outcomes for people with neurodegeneration. I think we do have the opportunity to reduce the global burden of dementia, and we do have the opportunity also to extrapolate this, looking at the unique chemistry for each of these disorders from macular degeneration, to Lewy body disease, to Parkinson’s, to ALS, to frontotemporal dementia, and we’ve begun to do this with what we call the arc trial, where we just as the arc was two by two by two, we look at people who have very, very early of these various things that we started with a macular degeneration, evaluate the larger dataset to look at what’s actually driving the process, and then look to see whether we can address those things that are driving the process and achieve better outcomes. Since so many of these have either nothing or virtually nothing that is helping prevention and reversal of that particular form of neurodegeneration.
So I think this is a very exciting time for all of us. Look forward to the upcoming trials on, not only our own trials, but those from multiple other laboratories. I look forward to an era in which we combine the pharmaceuticals that are targeting specific areas, specific contributors to the neurodegenerative process, and we combine these with precision medicine protocols, personalized protocols to get better outcome. We’ve been in an era of very much polarization, where people will focus only on single drugs or they’ll focus on more precision medicine type of protocols. I think that the time is really an exciting time to bring these together to get the best outcomes. So, I look forward to that. And with that I’ll end. Again, thanks to Aging for always looking toward the future, publishing exciting papers, and improving age-related diseases like Alzheimer’s disease. Thanks.
Click here to read the full study, published by Aging (Aging-US).
WATCH: AGING VIDEOS ON LABTUBE
—
Aging (Aging-US) is an open-access journal that publishes research papers monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].