The Longevity & Aging Series is a collaboration brought to you by Aging (Aging-US) and FOXO Technologies. This monthly series of video interviews invites Aging researchers to speak with researcher and host Dr. Brian Chen. Dr. Chen is an adjunct faculty member at the University of California San Diego and the Chief Science Officer at FOXO Technologies.
—
Below is a transcription of the fifth installment of the Longevity & Aging Series, where Dr. Amit Sharma from the SENS Research Foundation details a research paper he co-authored that was published by Aging (Aging-US), entitled, “Enhanced co-culture and enrichment of human natural killer cells for the selective clearance of senescent cells.”
Great. Thank you all for joining us today. We have a special guest. We have Dr. Amit Sharma, Group Lead at the SENS Foundation in senescence immunology research. Today, he’s going to be covering his recent publication in Aging titled, “Enhanced co-culture and enrichment of human natural killer cells for the selective clearance of senescent cells.”
Dr. Sharma, why don’t you start by giving a brief bio to the audience about your background, who you are.
Thank you, Brian. I started working as a group leader at SENS Research Foundation about 2 years ago. Before that, I was doing my post-doc at the Buck Institute in a project in collaboration with Dr. Judy Campisi and Dr. Pankaj Kapahi. We were looking at how DNA damage response can play an important role in aging and age-related diseases, and that’s how we got to cellular senescence.
I did my PhD back in India and my PhD was in understanding allergic inflammation and how microRNAs are important in regulating that. This, basically, project that I’m doing here at SENS in my lab, essentially I bring both of my experiences together by understanding SLS cellular senescence and how an aging immune system plays a role while regulating it.
Great. Maybe you can give us a little bit of background before your presentation begins about just the idea of using natural killer cells for anything really to control it as a biotechnology. Can you give us a little bit of background of what’s been done and what the idea is about around that?
Natural killer cells, as their name suggest, are a type of innate immune cells which can recognize specific type of receptors on the surface of any target. They play a very important role in regulating cells that are infected with viruses or pre-cancer cells or even cancer cells in our body and have been extensively studied and used for cancer immunotherapy.
Technology has gone leaps and bounds in that direction. They’ve shown that actually adoptive transfer of NK cells to patients seem to have a very beneficial effect in regressing tumors in various kinds of tumors. Lately they’ve found that adaptation of CAR-T technology where we can express a TCR to recognize a specific antigen on a cancer cell could be used to target them and NK cells allow us to expand that technology even more where you could essentially take an off-the-shelf NK cell, modify it and then use it essentially for many, many patients and it has none of the side effects of CAR-T technology.
I mean these are pretty exciting cells. I got specifically interested in NK cells since the original observation that they play an actually very important role in regulating senescent cells in our body as well. There’s plenty of evidence in that direction and I’m trying to understand how innate immune cells like NK cells are regulating senescent cells, what are the factors that regulate that interplay and how we can utilize them to develop novel therapeutic interventions.
Awesome. All right. Well with that introduction, why don’t I let you kind of walk us through your paper and then we’ll do a little Q&A afterwards?
Sounds good to me. Thank you. Let me start.
Thank you.
Again, thank you for giving me this opportunity to talk about my research in general and the kind of work that my lab is doing, and specifically the project that we published recently in the journal Aging.
My lab, as I said, is focusing on understanding and engineering innate immune system and regulating senescent cells as a therapeutic intervention. As you know, for the uninitiated, aging is an increasing risk factor for many diseases. That information has been out there and I don’t think there is much debate on that. We know that at least in developed countries, there is an increase in the number of people who would be over the age of 70. There is some data right there in the United States by 2023, one out of seven individuals will be over the age of 70. This is not only in the US, even in Europe we see that there is an increase in individuals over the age of 70. As we increase there is increased incidences of cardiovascular disease, dementia and cancer. Understanding how aging is regulating these processes could be very important in developing novel therapeutic interventions.
My lab is SENS Research Foundation and we consider aging interventions. Basically there are seven major forms of damages that occur and those could be repaired to possibly extend lifespan and improve health span. My lab focuses on targeted ablation of death resistance cells that we call senescent cells.
Again, for the uninitiated, what are senescent cells? Cells undergoing any kind of damage, internal or external, can lead to a cell cycle arrest. This arrest could be useful in tissue repair. We know that for repair after an injury and during embryonic development, this process is important and is vital. Most of those cells undergo apoptosis and they get cleared off and we do much better after that; however, some cells for certain reasons do not get cleared off. We’ll get into some of those reasons later on. We start seek an increase in senescence burden.
These cells or long term senescence cells tend to produce pro-inflammatory cytokines and chemokines, metalloproteases, also called SASP, which is known and has been shown to contribute to systemic inflammation as we experience in aging and also qualities of aging.
My lab mainly focuses on understanding why do we have increased senescence burden with age. We tend to consider these two be the main problems which is immune senescence, meaning the decline in immune surveillance that happens with age, and propagation of senescence as the idea is that as we have more senescent cells in our body, they tend to produce more SASP which can propagate a senescence. Those two factors contribute to aging and accumulation of senescent cells.
This brings us to the paper that we recently published here in Aging. To get you a brief overview of what we have known about how NK cells and senescent cells interact. NK cells are known to be one of the main drivers of immune surveillance senescent cells. That has been shown a couple of years ago. Briefly, natural killer cells express various kinds of receptors on the surface that recognize stress ligands on the target, like MICA. Once they recognize this receptor, they tend to scan the surface of the cell, in this case senescent cells, and they find that depending on the density of the activating receptor or inhibiting receptors like HLA-E, NKG2D or NKG2A are engaged. If there are more MICA receptors or activating ligands on the target cell, then natural killer cell releases these perforins and granzymes that can create pores in the target cell and cause apoptosis and they’re eliminated. However, it has also been shown that senescent cells find a way to escape this immune surveillance by increasing the expression of HLA-E, which binds to inhibiting receptor and block the release of these perforins and granzymes.
In addition to that, senescent cells release these perforin SASP factors. Some of them are chemoattractants for NK cells, but some of these factors include these soluble receptors like the extracellular domain of MICA is cleaved off by metalloproteases which can glue and bind to these activating receptors, blocking them, blinding them so these NK cells cannot recognize the targets and again, another way by which senescent cells can escape immune surveillance.
Better understanding of this process will allow us to develop novel therapeutic interventions. One of the things I’m not going to get into right now, we and others are trying to identify novel cynoantigens that can… This information then can be used to engineer CARs on NK cells to enhance the clearance of these cells and avoid this process which is also subject to age so we can overcome that. But in any case, understanding this interaction and the most optimal conditions by which NK cells can identify senescent cells and remove them is going to be vital for any future therapeutic intervention.
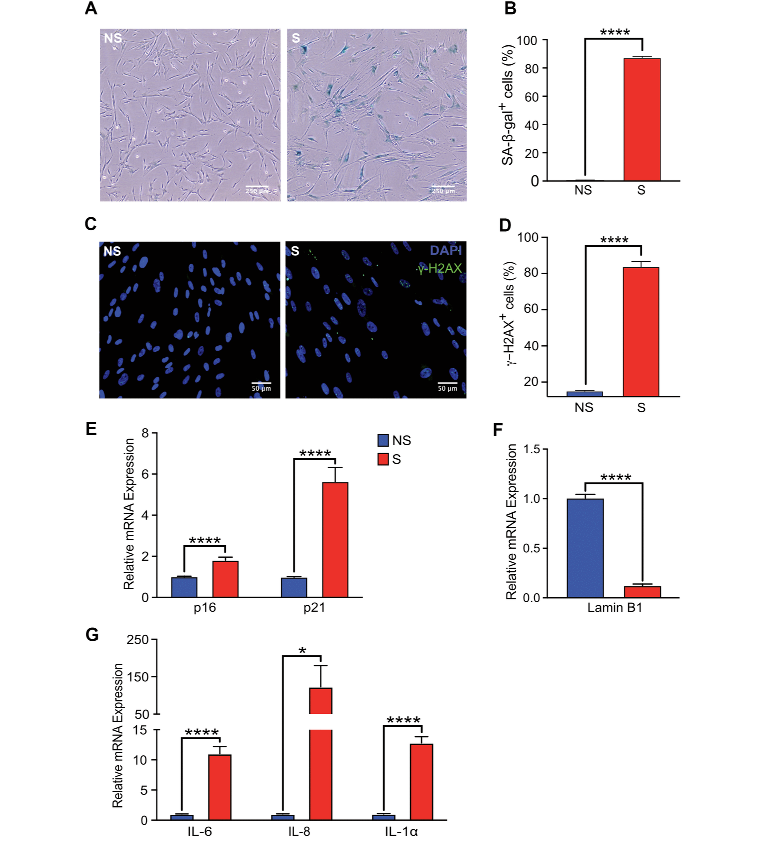
We started by establishing senescence model. This has been well established, well characterized by several other groups. Senescent cells show various markers and not one marker is sufficient to characterize senescence so we and others test other cells by establishing various markers to make sure our cells are truly senescent. These are for instance human fetal lung fibroblasts that were treated with doxorubicin. Pretty established model, and 10 days after doxorubicin treatment we measure the proportion of cells that express senescence associated activity and we find a significant increase in the proportional of cells that express that.
We also confirmed the expressional cell cycle checkpoint markers like p16 and p21, which seems to be significantly elevated in these cells and confirmed that by looking at the expression of Lamin B1, which indicates a loss of nuclear lamin and persistent DNA damage for S measured by gamma-H2AX and the expression of pro-inflammatory cytokines IL-6, IL-8 and IL-1a was also elevated in these cells.
Recently several papers have shown that actually senescent cells are quite different and they’re affected by the type of damage. We also, in this case, in order to study the interaction of NK cells with senescent cells, we also induced different kinds of damage. Here we are showing that we were able to induce senescence phenotype by radiating the cells with ionizing radiation with x-rays or treating these cells with etoposide. In either case we’ve seen an increase in proportional cells show and increase in SA-b-gal activity.
In addition to that we also measured the expression of main chemokines, CCL5, CXCL9, CXCL11 which are also the main chemoattractants of NK cells. We found that senescent cells tend to produce very high levels of these chemokines.
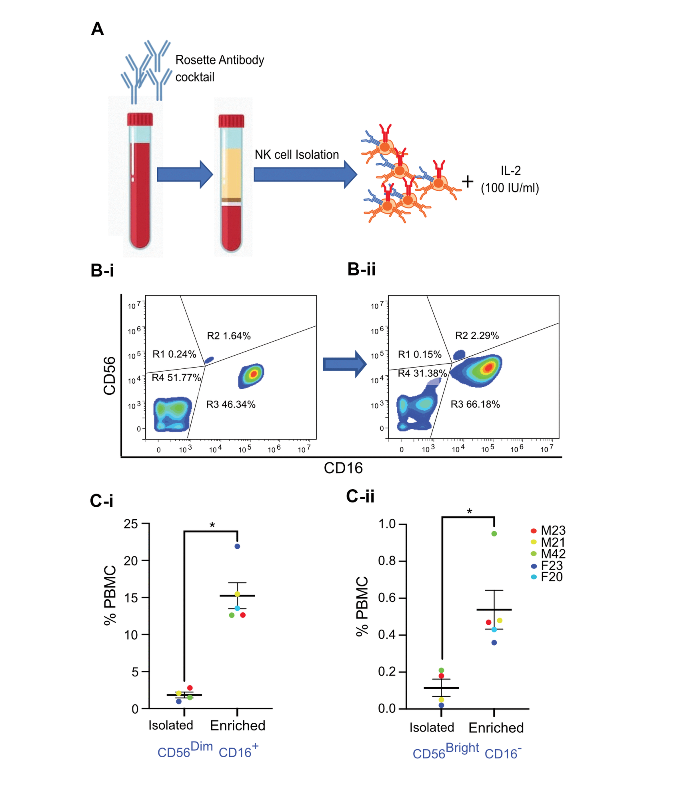
Now briefly, NK cells are not necessarily a monolith and at least in humans they can be characterized by the expression of at least three markers: CD56, CD16, and CD3. Absence of CD3 is essential because that way we can distinguish NK cells from T cells. These cells which do not express CD3 and express high levels of CD56, or we call them CD56 bright, and do not express CD16 are not responsible for cytotoxic function or ADCC function, but they tend to release alpha and gamma. Many consider these to be an early stage in differentiation of NK cells. The other population of NK cells that we frequently observe in peripheral circulation is CD56 dim NK cells that also express CD16 but they’re also CD3 negative. These are the ones which are mainly responsible for cytotoxicity and ADCC but they do not produce cytokine. But there is an interplay between these two cell types because the alpha and gamma produced by the CD56 bright NK cells tend to affect the cytotoxic function of CD56 dim NK cells.
We looked at the published literature and figured that the best way to study the interaction of NK cells with senescent cells was to first isolate NK cells, purify them and understand that we are working with a purer population of NK cells. We used rosette antibody cocktail which precipitates all immune cells in PBMC except NK cells which are present in the interface when we perform a density gradient centrifugation. When drawn with IL-2 at 100 IU/ml, we were able to expand and activate NK cells.
An example here, we’re showing that when we look at NK cells in PBMC based on CD56 and CD16 expression, we are able to identify both these subpopulations of NK cells, which after three days in culture after isolation we were able to expand them even more, both the subpopulations. Using this protocol we were able to show an expansion of both the population of CD56 dim, CD16 positive and CD56 bright and C16 negative NK cells from different individuals, both which were males and female from the age of 20 to the age of 42. We saw that using this protocol, I could isolate and enrich both these populations.
Now when we set up other co-culture, and we tried to keep the target to factor ratio pretty consistent, pretty physiological. Now, NK cells are known to be very, very effective in killing their targets as long as the targets are expressing the receptors, the activating receptors, on the surfaces. A single NK cell, it has been shown, is capable of killing up to five targets. All previous studies that have shown that NK cells can recognize and eliminate senescent cells have often used very high effector compared to targets, very high numbers. We wanted to keep it more physiological. We observed that when we keep one-to-one target factor we were able to see significant killing of senescent cells. But as we increased the factors, NK cells in here, we saw that the cytotoxicity towards senescent cells increased quite a bit but we also saw nonspecific killing increasing with increasing number of NK cells, or proportion of NK cells. This was observed 16 hours after co-culture of senescent IMR-90 or non-senescent IMR-90 with the primary NK cells.
We decided to stick to one-to-one target effect ratio that because by that we were able to achieve significant killing, pretty modest nonspecific killing of non-senescent cells. Using this protocol we were able to show pretty effective killing of senescent IMR-90 whether we use senescent by doxorubicin radiation or etoposide as measured by the LDH release assay.
Again, we were able to show that using this protocol we were able to achieve pretty effective killing once we isolated NK cells from different individuals, the cytotoxic function was preserved.
There is something interesting here that we found that the male NK cells were more effective in killing senescent cells but I mean the data set is too small and further analysis is required.
Most of these experiments that previously have been done to show that NK cells can kill senescent cells were done with freshly isolated NK cells and we were able to see cytotoxicity pretty well. But when we froze down these NK cells and revived them… And it has been shown that freezing of NK cells tend to reduce their cytotoxicity and the ability of NK cells to kill senescent cells even after reviving is essential because if any therapeutic intervention has to occur then we will most likely be working with the cells that has to be frozen down. We found that in our case the cells that we have isolated using our protocol seems to retain this cytotoxic function even after freezing.
This next set of experiments is pretty interesting because this really was essentially a mistake. The student who was working on this project, the first author on this paper, she one time forgot to remove the plate after setting up the co-culture after performing the assay. When we came back after the weekend we looked at the plate, we observed that after four days in co-culture most of the non-senescent cells were still fine but these NK cells continue to kill senescent cells. We were able to see pretty low numbers of surviving senescent cells in co-culture.
This was the case with doxorubicin treated senescent cells or whether we do senescent by radiation or etoposide treatment. We continued to see a decline in the numbers of senescent cells whereas non-senescent cells tend to survive really well even after four days in co-culture. And this was measured by Calceim assay where we were just measuring the surviving cells on the plate. We chose to do this instead of the LDH release assay because LDH after release is not very necessarily very stable and it has a half life of 16 hours. It tends to degrade after that. We decided to just count the number of cells that are still attached to the plate.
Here is the image of what we normally see after 16 hours of co-culture with of senescent cells with NK cells. Whereas non-senescent cells pretty seem to survive really well the co-culture, we tend to see that after 16 hours of co-culture there are only fewer senescent cells surviving. If we allow this culture to go on longer, then we see almost all senescent cells are gone, which we think is pretty interesting and we are following up on this. We’re trying to understand the differences between these senescent cells that are killed after 16 hours of co-culture versus the ones that cannot survive after longer co-culture. We’re trying to understand the difference between these two cells. That probably will form another way we can target these cells.
With that, I’d like to acknowledge my team who participated in this work. Tes is the postdoc who contributed significantly in this study. Kristie is the first author. Yafei has just joined our lab, he didn’t really participate in this work. Gina also initially helped us with this project, actually is a master student who’s pursuing another way by which we are identifying service receptors. She didn’t participate in this project but she has been pretty helpful.
With that, I’ll thank you and Aging for allowing me to talk about my research here. I’ll take any questions.
Great. Unfortunately you’re only stuck with my questions but thanks for the presentation.
First, I might have missed it but can you kind of walk generally high level what is the procedure on isolating or enriching for the NK cells?
We use a cocktail of antibodies which is designed to precipitate cross link with RBC and precipitate all cells except NK cells in the co-culture. When we perform a density gradient centrifugation with PBMC, we can use histopaque over the blood and we spin it down, we can get PBMC. But if we pre-incubate the blood with this cocktail of antibodies, everything will precipitate and the only thing that would be in the interface would be NK cells.
These NK cells are pretty pure, quite enriched. They are very high proportion of CD56 expressing cells. If we just look at that, they’re CD56 positive, they’re CD16 low, or I mean… They are CD16 positive and they don’t have CD3 expressions. We’ve seen by simply doing this initial isolation step, we remove all contaminating cells.
Some papers have shown that actually these cells are pretty good in removing senescent cells but they have either worked with NK cell line or they worked with PBMCs. PBMCs tend to contain CDAP cells and TDAP cells can attack other cells as well non-senescent as well so they tend to have a higher cytotoxicity towards non-senescent cells so they end up doing a very shorter co-culture, which again is not necessarily very physiological.
Pardon my ignorance, but I’m just trying to think in practice if this ever reaches the clinic, which certainly seems very promising, do we need to do HLA matching like we would do for bone marrow? Or is this you can farm a whole bunch of NK cells from people and use it just indiscriminately?
There are three possible avenues this can go in. One that we are also exploring is to get fetal NK cells and use them as off-the-shelf NK cells and relatively in patients who are having a disease phenotype which is affected by senescence burden. Those cells could be very effective in removing those.
We could also talk about isolating NK cells from blood relatives and then doing an adoptive transfer to remove senescent cells.
And another thing that can be done is that we can haplo match NK cells and make a blood bank… I mean, a bank of NK cells which can basically cover the entire human population and then we could partially match them and then do a transplantation that way.
Got it. Sticking on the whole theme of how this might play out clinically in the future, on the issue of target to effector ratio, I know you tried to keep it one-to-one in your case, but I could also imagine if you’re in a situation of a child, let’s say, where there might not be that many senescent cells, it might be good to keep a low ratio but if you are in a isolated situation, let’s say for osteoarthritis or something where you might want more bang for your buck, do you think that you would then want to tune that target to effect a ratio to be a little bit more, I guess, efficient and more aggressive I guess?
Yeah, that is true and also I think we can also design NK cells to recognize unique attributes on the surface senescent cells. There are several groups who are made significant progress in this and we are also working on this, that we can actually design these NK cells to express CARs towards a target so that way we can still limit the target effective ratio and then we can get very high effective connect.
Yeah. Your question actually made me think of another question around senescent cells. Are senescent cells all the same or are there actually different flavors of senescence?
It’s been shown that there is different flavor of senescent cells. Depending on the cell of origin and the type of insult that is causing senescence, they actually have a profile quite different.
Another thing that we are looking into is that primary senescent cells and paracrine senescent cells are also different in terms of gene expression. We’ve also observed it. We are looking to publish that pretty soon. Also it has been shown that within a population of senescent cells, at least in vitro we know that by performing single cell analysis, they are quite different.
Yeah, there is significant variability on these cells but what we know is that almost 90% of senescent cells express one or the other stress marker like MICA on the surface. It is still possible for NK cells to recognize them all and kill them.
Another ignorant question for me around senescent cells, but at the earlier slide you described all the different defenses that a senescent cell has to block NK cells. What’s the reason behind having those in your thinking? It seems like it definitely evolutionarily a cell probably wants to preserve itself, but from the standpoint of an organism that might not make sense. Am I thinking about that incorrectly?
No, you’re absolutely correct and that is a good question. We are also looking into that. I think partly the expression of inhibitory receptors on the surface like HLA-E could be a way by which a cell is actually calibrating the immune surveillance function by NK cells, meaning it probably… That gives that cell more time to repair whatever damage it has and so it’s not eliminated prematurely just by expression of stress receptors on this surface.
The release of these soluble proteins or these surface receptors like MICA or soluble MICA to block NK cell function possibly could be a byproduct of modified matrix of senescent cells where there are several proteolytic enzymes that are just active on the surface and they just cut off these surface proteins and release them.
It could be both of those things. It could be a way by which cell has evolved ways to just prevent itself from dying and other could just be a byproduct of inflammation.
Right. Certainly would make sense that… The latter could make sense in that biology maybe hasn’t thought that far ahead and this is just the way it is. It doesn’t have to make sense necessarily.
Exactly. We also know that the ability of NK cells to function appropriately also declines with age. We quite don’t understand why that is and we are looking into that as well. But yeah, I mean that also happens. I mean, that the ability of our immune system also declines. In addition to the fact that senescent cells are mounting these ways to prevent care, the immune system also is not as effective as we age.
That’s why you alluded to fetal sources of NK cells as potentially nice to have, which makes me think now we’re having the hetero chronic parabiosis folks meeting the NK cell based therapy folks.
Yeah.
That’s nice. So big picture, where are we in the development of NK cell based therapies for senescence right now? Have there been in vivo studies at all? Do we know that tolerance of an in vivo organism to such a therapy?
We are starting it. I mean, there is so much information from cancer that we can learn that will be useful for us when we are talking about any NK cell based therapy. They have done adoptive transfer in mice. They’re pretty effective. They’re pretty well tolerated in patients as well, at least in context of cancer.
What we are doing is we are isolating NK cells. We’ve done that from young and old mice and we are trying to see if there is an age related decline in immune function of NK cells that may be contributing, especially when we isolate these NK cells, enrich them in vitro and then try to transplant them back in old mice or doxorubicin treated mouse models to see how that affects senescence burden. That I think would be the basis of any further clinical work that goes in that direction.
Makes sense. Last question to you. Bigger picture of what are some bottlenecks? Every time I’m involved in research there’s always one piece of knowledge or funding. You know what to do, but you just don’t have the funding, or maybe the technology is limited. What do you think are the biggest bottlenecks in driving this technology forward?
I still think that we do not completely understand the surface of senescence cells and how heterogeneous these cells are. That’s what we are trying to focus on and that’s where really the bottleneck is moving forward. There is a case to be made that they’re so different, these cells, senescent cells, depending on those various factors we discussed that they may not… It is possible that we may not have one way to target them. There may be such variation in these cell types that we may have to think of multiple ways to look into these cell types.
The other bottleneck is getting clinical sample. I think most of the work so far, and we are kind of guilty of that as well, is being done with cells which are grown in culture, adaptive to grown in culture. I think more work has to be done on primary tissue, which is not that easy to come by.
Unless you’re doing it in a mice, I suppose.
Well mice, the interesting thing about mice is NK cells in mice and humans are very, very different and they express very different receptors. We’ll have to very seriously think about how to go about that. I mean, that’s why I talked about how we could maybe use primary human tissue. I’m very interested in looking at these organ-on-chip models as an alternative to most models as well.
Got it. That makes sense. Yeah, I’m happy to donate all of my senescent cells and tissues to you for your research, so…
I appreciate that.
Let me know.
All right, well thank you for walking us through this very exciting paper. Best of luck to you and your lab and the whole field for driving this forward. It seems very exciting. I hope that it comes to fruition within my lifetime so we could all benefit from it.
Thank you so much for talking to me about this and giving me a chance to explain my research a little bit more. And thank you to the journal Aging for actually allowing us to publish. This was my first corresponding author person so I’m very glad that I was given this chance. This will open up a lot of doors for me.
Click here to read the full paper published by Aging (Aging-US).
Longevity & Aging Series Episode 4: Drs. Carly Bobak, Cristian Coarfa, and Andrew DiNardo
Longevity & Aging Series Episode 3: Dr. Steve Horvath – Epigenetic Clocks
Longevity & Aging Series Episode 2: Dr. Steve Horvath’s Special Collection in Aging
Longevity & Aging Series Episode 1: Drs. Alex Zhavoronkov and Frank Pun
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
—
Aging (Aging-US) is an open-access journal that publishes research papers bi-monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].