Dr. Ya-Jen Chiu from the Department of Life Science at National Taiwan Normal University in Taipei, discusses a research paper she co-authored that was published by Aging (Aging-US) in Volume 14, Issue 18, entitled, “Novel TRKB agonists activate TRKB and downstream ERK and AKT signaling to protect Aβ-GFP SH-SY5Y cells against Aβ toxicity.”
Behind the Study is a series of transcribed videos from researchers elaborating on their recent studies published by Aging (Aging-US). Visit the Aging (Aging-US) YouTube channel for more insights from outstanding authors.
—
Hello, everyone. My name is Ya-Jen Chiu, and I am a postdoc at the National Taiwan Normal University at Dr. Guey-Jen Lee-Chen’s Laboratory. My background is in neuroscience with a focus on drug discovery and molecular mechanisms for treating neurodegenerative diseases. I would like to present you today our paper recently published in Aging-US journal, Volume 14, issue 18, entitled, “Novel TRKB agonists activate TRKB and downstream ERK and AKT signaling to protect Aβ-GFP SH-SY5Y cells against Aβ toxicity.”
Alzheimer’s disease, in abbreviation AD, is the most common form of dementia, and Aβ activation is one of the early events in AD pathology. The accumulation of misfolded Aβ proteins causes neurotoxicity which leads to apoptosis. As a result, clearing of aberrant Aβ aggregates has been considered as a therapeutic approach to moderate disease development in AD.
TRKB receptor activation has been linked to improved memory formation and storage as well as neuroplasticity, neurogenesis, neuro outgrowth and neuron survival. Upon TRKB receptor activation, ERK and AKT phosphorylates CREB to activate transcription of genes implicated in these functions, such as BDNF and Bcl-2. Previous studies have shown that BDNF transcription and protein expression are reduced in the hippocampus, cortex, and Meynert basal ganglia of AD brains. Several preclinical studies using different AD mouse models have shown that enhanced BDNF expression protects neurons and improves cognitive performance.
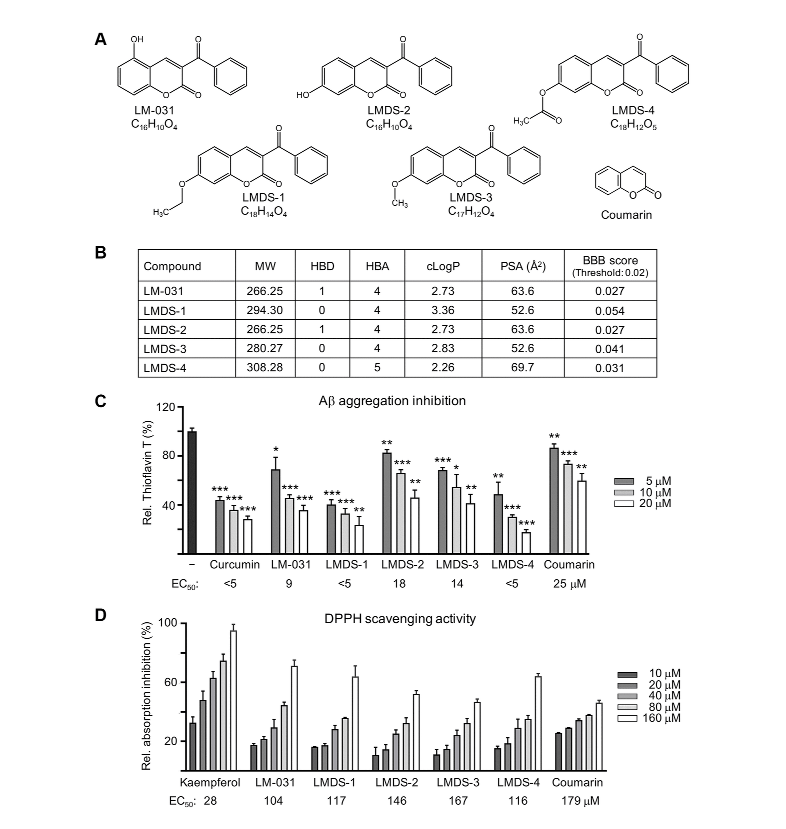
However, the poor bioavailability of BDNF such as the short half-life in plasma and the limited blood-brain barrier permeability limits its use. So the discovery of new small molecule agonist of the TRKB receptor is a potential therapeutic strategy for neurodegenerative diseases. A previous study has found that another coumarin derivative, LM-031, has had neuroprotective benefits in Aβ-GFP SH-SY5Y cells, while up-regulating the CREB, BDNF, Bcl-2 pathway.
In this study, we used chemical similarity search software tools to [inaudible] for LM-031 and analogous compounds and discovered four top-scoring compounds, LMDS-1 to 4, as possible TRKB agonists.
As shown here, all LMDS compounds showed potential for binding to TRKB domain d5 by docking computation. Additionally, all LMDS compounds inhibited aggregation and provided a neuroprotection including for [inaudible] and reducing the reactive oxygen species, caspase 1 and acetylcholinesterase, activity in SH-SY5Y cells with infused Aβ-GFP expression.
Furthermore, TRKB knock-down reduced downstream ERK, AKT, CREB signaling, and a newer outgrowth for multi-effects of these LMDS compounds. Analogs LMDS-1 and 2 were further examined following treatments of ERK inhibitor U0126 or PI3K inhibitor Wortmannin. And the result demonstrates that the neuroprotective benefits of LMDS-1 and 2 are mediated by activating TRKB downstream ERK, PI3K-AKT, and CREB signaling. When effects of LMDS-1 and 2 signaling were compared with BDNF, there is no significant difference in extent of ERK, AKT, and CREB activation between BDNF and LMDS-1 or 2.
Considering BBB impermeability is a P obstacle in treatment of brain disorders, we tested the BBB permeable of LMDS-1 and 2, and both compounds were found to be BBB permeable by a parallel artificial membrane permeability assay.
Now we come to our main study findings. What we found is that two LM-031 and analogous compounds, LMDS-1 and 2, may serve as the potential TRKB agonist to treat AD. This is supported by our results in Aβ-GFP SH-SY5Y cells. We show TRKB signaling activation, aggregation inhibition, oxidative stress and caspase 1 and acetylcholinesterase reduction as well as neuro outgrowth promotion. Application of LMDS-1 and 2 to AD animal models [inaudible] to confirm the neuroprotection effects. Additionally, it will be informative in the future to study the pharmacokinetics of LMDS-1 and 2 to provide insights for clinical studies.
I will end my presentation by acknowledging Molecular Imaging Core Facility of National Taiwan Normal University, National Core Facility of Academia Sinica for the technical assistance, National Center for High-Performance Computing for providing computational results for docking computation.
Also, I would like to thank Aging-US journal for the opportunity to present our work. Thank you for your attention.
Click here to read the full study published by Aging (Aging-US).
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
—
Aging (Aging-US) is an open-access journal that publishes research papers bi-monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].