Dean Bunnell, PhD candidate from the Department of Biological Sciences at the University of Alabama, describes a research paper he co-authored that was published by Aging (Aging-US) in Volume 15, Issue 6, entitled, “RNA virus-mediated changes in organismal oxygen consumption rate in young and old Drosophila melanogaster males.”
Behind the Study is a series of transcribed videos from researchers elaborating on their recent studies published by Aging (Aging-US). Visit the Aging (Aging-US) YouTube channel for more insights from outstanding authors.
—
Hi, my name is Dean Bunnell. I am a PhD candidate in the Chtarbanova Lab at the University of Alabama. I would like to thank the journal of Aging for the opportunity to make this video for a recent publication titled, “RNA virus-mediated changes in organismal oxygen consumption rate in young and old Drosophila melanogaster males.” I’m a first co-author on this paper and I’m grateful to have worked alongside co-author Eli Hagedorn.
This study was inspired by our previous work, in which we showed that infection of Drosophila melanogaster with the RNA virus, Flock House virus, or FHV, results in significantly increased mortality of older flies in comparison to younger flies. This appears to be the result of an impairment of a mechanism of disease tolerance with aging because there were no significant changes in virus load between age groups. This work also showed that aged flies display differential regulation of metabolism genes after FHV infection.
Aging is accompanied by increased susceptibility to infections, resulting in higher morbidity and mortality in the elderly. One hallmark of aging is mitochondrial dysfunction, which has direct implications for host metabolism and could also affect the hosts’ ability to mount an effective immune response, as immune activation is an energetically costly process.
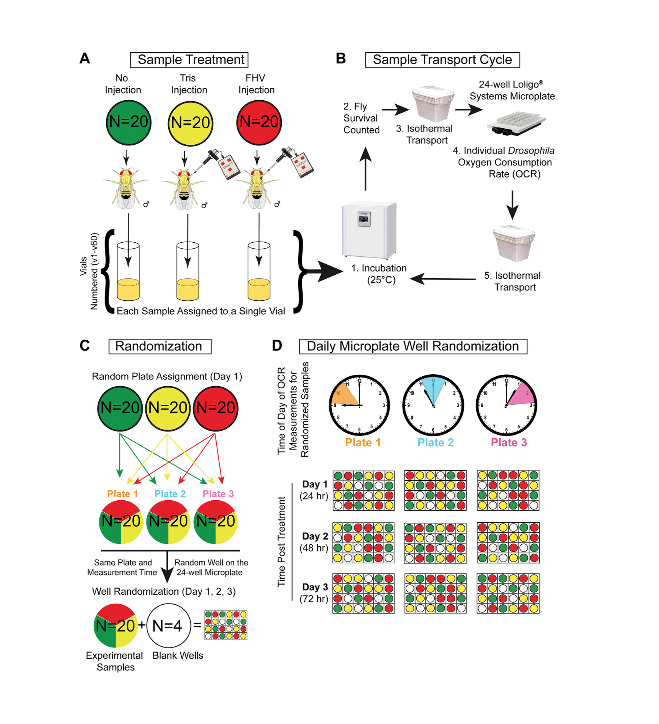
In the current study, we hypothesize that organismal metabolic rate is deregulated in drosophila following FHV infection and that age-dependent differences in metabolic rates following infection could influence older hosts accelerated mortality. To test our hypothesis, we conducted longitudinal single-fly respirometry with the Loligo Microplate System to measure oxygen consumption rates, or OCR. This system utilizes an oxygen sensor within each well to detect changes in the volume of oxygen, enabling the calculation of individual organismal OCR.
Most organisms’ oxygen consumption occurs in mitochondria, so OCRs can provide an indirect measure of mitochondrial function. Despite many well-established protocols to assess mitochondrial function, these techniques destroy the sample and do not allow longitudinal assessment of the same individual. This makes our approach unique, as it enables longitudinal measurements of an individual’s metabolic rate in the course of an infection. By recording the survival status of each individual prior to each measurement, we were able to determine whether variations in OCR throughout the experiment influence organismal survival of FHV infection.
We found that FHV infection significantly reduced organismal OCR compared to Tris controls, confirming our hypothesis that FHV alters the host’s metabolic rate. The longitudinal assessment showed that across time points, young flies are able to modulate their OCRs, while aged flies are not. The young fly’s ability to modulate OCR is representative of energy conserving hypometabolism, which has been shown to promote disease tolerance in mammalian models of bacterial infection. This is consistent with our previous findings that age-dependent impairment of disease tolerance is responsible for the accelerated mortality in older flies.
We observed a significant difference in OCR at 24 hours between young and aged flies, so we investigated whether OCR differences at this time point could affect infection outcomes. FHV-injected flies that survived the experiment had significantly higher OCR at 24 hours than flies that died prior to the 48-hour time point. This demonstrates that organismal OCR could be a factor that impacts infection outcomes.
Based on the results from our study published in Aging, we are excited to propose some future directions that our research could take to investigate the interaction between older hosts and viral pathogens. For example, future studies can utilize high-resolution respirometry on isolated mitochondria to complement this study and further analyze the effects of FHV infection. This will enable an assessment of the functionality of each complex in the electron transport chain, and could provide a more direct assay of mitochondrial function.
Additionally, we are interested in further characterizing the difference that we observed in OCR modulation between young and aged flies in response to FHV. For example, we would like to know, what are the mechanisms that occur with aging that result in older flies losing the ability to decrease their metabolic rate after infection? It would be of particular interest to identify factors associated with this process that could be manipulated in older flies to mitigate the consequences of infection.
Finally, I would like to thank all co-authors on this study, Eli Hagedorn, Beate Henschel, Daniel Smith Jr., Stephanie Dickinson, Andrew Brown, Maria De Luca, Ashley Turner, and Stanislava Chtarbanova for their dedication and contributions to this work.
Click here to read the full study published by Aging (Aging-US).
—
Aging (Aging-US) is an open-access journal that publishes research papers bi-monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].