Dr. Velia Fowler and Dr. Catherine Cheng, formerly from The Scripps Research Institute, discuss a 2019 research paper they co-authored that was published by Aging (Aging-US), entitled, “Age-related changes in eye lens biomechanics, morphology, refractive index and transparency.”
Behind the Study is a series of transcribed videos from researchers elaborating on their age-related studies published by Aging (Aging-US). Visit the Aging (Aging-US) YouTube channel for more insights from outstanding authors.
—
Hi, I’m Velia Fowler. I was a professor at the Scripps Research Institute for many years and Cathy Cheng was a senior post doctoral research associate in my lab and became a staff scientist when she was working on this project. She now has advanced to become a tenure track Assistant Professor at the Indiana University School of Optometry in Bloomington, Indiana, and I have since then also moved. I’m now a Department Chair of Biological Sciences at the University of Delaware. So we’ve used this study to help move our careers along and to start new avenues of investigation in this area.
And I’ll now turn it over to Cathy to tell everyone how we got started on this project.
So this project really originated from Velia and my interest in aging in the lens. So one of the pathologies that almost everybody will face originates from the lens. There are two age-related pathologies that we’re really interested in studying, including cataracts, which is any opacity in the lens, and cataracts are the leading cause of blindness in the world and it is the number one vision-related Medicare cost in the United States. Cataracts can be alleviated by surgery, but the access to surgery is not equal worldwide. So despite all of our efforts, we still don’t know what causes cataracts, and in particular, we were interested in understanding how age-related cataracts work.
The other lens age-related pathology is presbyopia. Presbyopia is the inability of your lens to change shape so that you can see up close. So everybody needs reading glasses, and this is due to partially the growth of the lens throughout your lifetime, as well as the increased stiffness in your lens throughout your lifetime. And again, it is unknown what are the cellular and molecular mechanisms for increase in lens stiffness with age.
We embarked on this study really to try to understand what were the driving factors for changes in the normal lens during aging, and we used a mouse model because mice age much quicker than primates and it serves as a way for us to understand what happens to the lens during normal aging. Previous studies have extrapolated data from younger lenses and made predictions about the older lens and we found several things that were different in our aging study.
Dr. Velia Fowler
So in order to study lens aging in the mouse, we needed to assemble a team of experts from around the world so we could study all the relevant cellular and biological physical features of lens aging with relevance to cataracts and presbyopia. In my own lab, we recruited another postdoctoral fellow, Justin Parreno, to help out with the lens dissections, lens imaging, and the biomechanical measurements, as well as Roberta Nowak, our research assistant who assisted with managing the mouse colony, which got large as they have to be aged, which is a very long time for up to two years or over two years.
Then we needed a team of microscopists to do the electron microscopy to look at the ultra structure of the lens cells because there’s no other way to look at lens cells in the middle of the lens unless you do electro microscopy, which is a process where we can fix the lens well enough to get the fixatives to penetrate throughout the entire lens, which in an older mouse is millimeters thick. This included Sondip Biswas and Woo-Kuen Lo at the Morehouse University School of Medicine. We also needed a team of investigators to study the refractive index of the lens as the lens aged. So this, again, is a very specialized technique and the investigator we recruited, Barbara Pierscionek in the United Kingdom, collaborates with a group in Japan at the Synchrotron, I think its the Riken-8 Synchrotron. Is that right, Cathy?
Dr. Catherine Cheng
Yes.
Dr. Velia Fowler
Yeah, the Riken-8 Synchrotron, to do soft x-ray defraction where they can image entire mouse eyes without perturbing them, so non-invasively image the entire mouse eye to look at the refractive index of the different tissues and they can then segment the images to see what the refractive index of the lens is. And that team includes, and this is where Kehao Wang … Cathy, you’re going to have to tell me, I can’t remember.
Dr. Catherine Cheng
Kehao is Barbara’s postdoc. And the other three Japanese scientists are the ones who did the experiments at the Synchrotron.
Dr. Velia Fowler
Oh, okay. So Kehao Wang is postdoctoral fellow with Barbara Pierscionek, assisting with analyzing the data from the soft x-ray defraction imaging of the mouse eyes. And then the three scientists from Riken-8 are Masato Hoshino, Kentaro Uesugi, and Naoto Yagi and they operate the soft x-ray defraction aspect of the synchrotron and were totally essential for this project. And finally, we also, really late in the project, learned that Juliet Moncaster had some similar observations on a different strain of mice, which we were able to then incorporate into our manuscript to show that the phenomenology that we discovered in the aging mouse lens was not specific to one strain of mice. So that’s a very important finding as well.
So, as you all can hear, this is an international team of investigators from the United States, United Kingdom, and Japan in order to solve the problem of what happens during mouse lens aging. Now, Cathy will describe what we actually did to study the lens. What did we learn?
Dr. Catherine Cheng
So, the lens is a really interesting aging model because the lens retains all of the cells that were ever made within the organ. So, the cells in the middle of your lens have been there since you were an embryo and the lens continues to add more layers on the outer cortex throughout your lifetime. We’re setting both cells that are chronologically old, so aged cells, but we’re also setting cells that were made later in life. This makes this tissue a unique aging model that we can study multiple generations of cells all within one tissue sample.
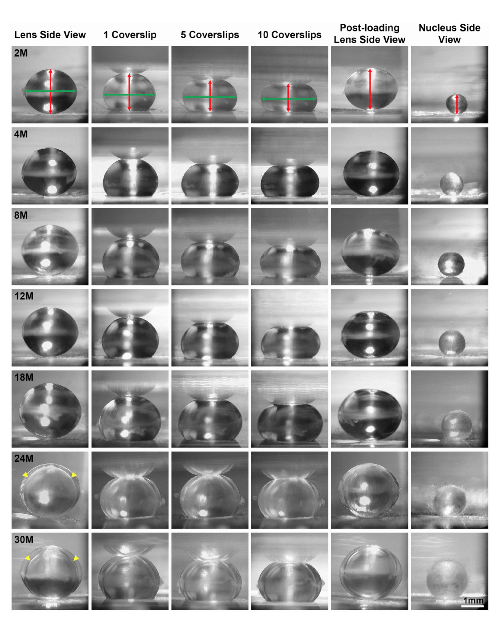
One of the first things that we did was look at the stiffness and the morphometric features of the lens through aging. We looked at mice that were between two months all the way up to 30 months of age, and in the black six mouse strain, 30 months of age is approximately the maximum life span of these mice. We basically covered a lot of territory, so from young adult mice all the way up to very old mice, and we were fortunate to receive some of the older mice from Dr. Bill Balch at the Scripps Research Institute. He was very generous in providing us with his aged mouse colony and we were able to get those eyes and lens samples from his mouse colony.
Our studies found that the stiffness of the mouse lens increases with age and it continues up through 30 months. This is similar to a primate lens, so we have similar findings as primate lenses because we know that there’s increased stiffness of human lenses with age as well. We found sort of surprisingly that the lens stops growing, at least in the mouse, around 18 months of age. So the lens doesn’t increase much in size and weight as the lens ages, even though there’s an increase in stiffness.
We think that the stiffness of the lens cannot be directly attributed to the increase in lens size. So this is something that had been a longstanding hypothesis that the growth of the lens is what is causing the increase in stiffness, but at least in the mouse, we don’t see that as a direct correlation. We continued the study by having Justin Parreno look at the different morphometric properties of the cells of the inside the lens.
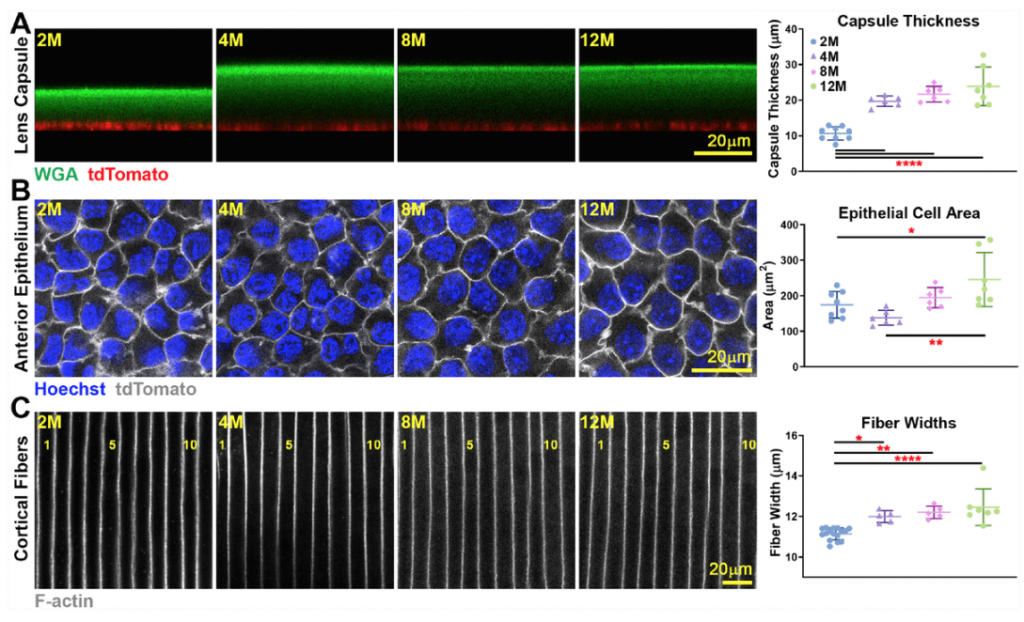
The lens is also encapsulated by a membrane called the lens capsule, and we measured the lens capsule thickness, we looked at the size of the epithelial cells and the fiber cells, and we found no significant changes past around four to eight months of age. These parameters did increase slightly between young mice at two months, but up to about four to eight months, we don’t see any more increase in the thickness of the capsule or the size of the cells.
We think that the increase and lens stiffness also doesn’t have much to do with sort of changes in the cell shape or size or the thickness of the lens capsule that surrounds it. So we still don’t have a great explanation for the increase in lens stiffness, but there is one parameter that does increase with lens stiffness and with lens age continuously and that is the increase in the size of the lens nucleus. The lens nucleus is the center of the lens and this sort of hard central part of the lens does increase in size with age, so it’s possible that the increase in the lens stiffness may be partially related to this increase in the size of the center of the lens.
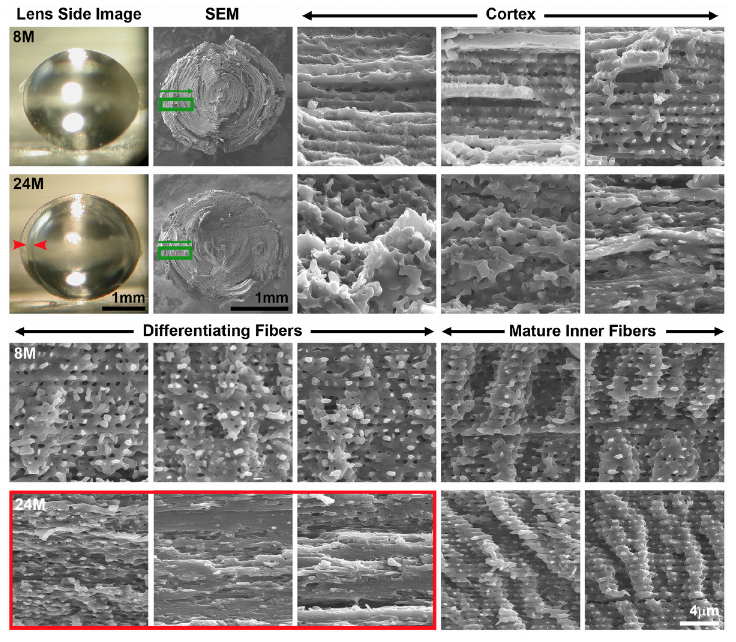
Our collaborators at Morehouse, Dr. Lo and Dr. Biswas did the electro microscopy. One of the reasons why we did that part of the study was because we noticed that a lot of the lenses over the age of 12 months had a cortical cataract. This is in the cortex of the lens, the periphery of the lens, and we could see a very distinct ring in that region. In order to investigate that, Dr. Lo and Dr. Biswas did electro microscopy and we were able to find that there is a zone of compression of the cells, so there’s a collapse in those cells and this causes an optical discontinuity leading to the cortical ring cataract.
Another age-related cataract that we often saw in the mice were anterior cataracts right at the anterior pole of the lens, so I did the whole lens staining and imaging to look at that defect and we found that there is an incomplete elongation of the cells within them that make up the lens. And this leads to the anterior cataracts. So sort of a surprising discovery was that the age-related cataracts in mouse lenses is due to structural changes inside the lens. So this is a little different from when we think about humans who have nuclear cataracts in the center of their lens and a lot of times that’s attributed to a protein degradation or protein aggregation.
It’s possible that because the mice are raised in an indoor environment, it’s a little bit different than the aging that occurs with humans and other larger primates who are exposed to sunshine and other types of UV and other sources that may lead to oxidative stress and protein aggregation. That was sort of an unexpected finding for us.
Then Dr. Pierscionek and her collaborators in Japan and her postdoc in the UK provided us with beautiful refractive index data, and our findings show that the mouse lens has a two-tiered refractive index gradient. So this is a little different also from other lenses that she’s studied. She’s looked at primate lenses, cow, rabbit, zebra, fish lenses even. The mouse lens has this two-tiered refractive index radiant that kind of looks like a two-tiered cake and we discovered that the refractive index in the mouse lens peaks at about six months of age, but the area of highest refractive index does increase with age. It expands and it correlates almost exactly with the size of the lens nucleus, which is the hard center of the mouse lens.
Her team in Japan imaged mice between two months and 30 months of age and got us all this really incredible data and this helps us to understand how the refractive index changes with age and we expect sort of similar changes in primate lenses so that’s something that I’m sure she and others are interested in working on.
Dr. Velia Fowler
Okay. So first of all, when I moved to University of Delaware, Cathy Cheng had already gone to Indiana University, but Justin Parreno moved with me to help set up my lab and continue our collaborative studies on lens biomechanics and aging. And so the direction that my lab is really interested in is to look at the cellular changes within lenses as they’re changing shape under conditions that mimic accommodation, a mimic to change in the shape of the lens that leads to either focusing near or far, so we’re using the mouse models to develop our imaging methodologies and to be able to understand what might be the genetic basis of the lens’s ability to change shape and how that changes with age. So there’s the aging connection there.
Then we are also starting to work on the nonhuman primate lenses to understand whether the cells in the primate lens change their shapes and are important to change their shapes in order to allow the lens to accommodate since non-human primates such as these, their lens will accommodate a change of shape to focus near and far, whereas the mouse lens remains round. The mice pretty much look at very close objects. They rely more on their whiskers and their sense of smell and their hearing than they do on their eyes to detect things in their environment.
So my lab is more moving in the direction of sort of bioengineering, biomechanics of lenses comparing the mouse and the human, but with this emphasis on how the cellular structures contribute to the flexibility of the lens in young lenses and change to lead to lens stiffness during aging.
Cathy is, I believe, taking a slightly different direction here.
Dr. Catherine Cheng
So I set up my lab at Indiana University just a little over a year ago. My interest actually now has shifted to focus on Ephrin signaling within the lens, and I’m still working on the aging aspect because in humans mutations in EPHA2 and Ephrin A5, one is a receptor and the second is a ligand, lead to age-related cataracts, as well as dominant and recessive congenital cataracts. I’m really interested in understanding what happens in humans who have mutations, especially in EPHA2, that leads to these age-related cataracts. My recent work has shown that in six-week old EPHA2 knockout, the mouse model, we can find that they have early onset cortical cataracts, cortical opacities, so I’m really interested in studying that because this mirrors the human cataracts, which are also cortical, or in the periphery of the lens.
I’m embarking now on an aging study of the knockout mouse lens that EPHA2 or Ephrin A5 knocked out and really understanding what happens to those lenses with age, and one of the interesting findings recently is that these two knockout lenses don’t have a decrease in the overall lens stiffness. We do find that they are more resilient, so they are more elastic and they bounce back after the compression better than the wild-type lenses. This is an unusual finding and I’m working on sort of an imaging study to try to determine what is the cause of that change, so really trying to understand what imparts the elasticity and sort of the biomechanical properties of lenses. Those are sort of the short-term directions, but long term I would like to age the EPHA2 and the Ephrin A5 knockout mice similar to what we’ve done with the wild-type and really look at what happens to the lens in these knockouts with age.
Dr. Velia Fowler
If I could add, I think Cathy’s research direction really illustrates where the field wants to go, which is to identify the molecular correlates or causes of the cellular and the structural changes in the lens that accompany aging, and then if you understand that, you might be able to intervene. But right now we’re still at very early days, probably more than Ephrin and EPHA2 receptors involved. There’s going to be a lot of molecules, but I think her work is really just going to be the tip of the iceberg and I think we can all look forward to learning more about the molecular basis of aging now that Cathy and myself and our team have established kind of a roadmap of what to look for, which wasn’t available beforehand.
In conclusion, in summary, what I’d like to summarize about our contribution is that this is the very first comprehensive analysis of aging in the mouse lens, starting from very young mouse, one to two months old, all the way up to over 30 months old, which is a very ancient mouse. The mice are not happy when they’re that old. We really looked at all the cellular and structural events that happened during aging, including the cell shapes, the sizes, the locations, the capsule thickness, the refractive index, so the functional properties of the lens, in other words, the optical properties, the refractive index, and mapped it onto the structural features of the lens, as well as the biomechanics.
Through this process, we discovered some unexpected findings which Cathy already summarized for you earlier, and I think this is then what allows us to have a roadmap for the future to link molecular changes to the cellular and structural changes during lens aging. I hope to see many more follow-up studies from many groups in the future on this topic.
Click here to read the full study published by Aging (Aging-US).
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
—
Aging (Aging-US) is an open-access journal that publishes research papers monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].
